| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:44:38 UTC |
|---|
| Update Date | 2016-11-09 01:19:17 UTC |
|---|
| Accession Number | CHEM031560 |
|---|
| Identification |
|---|
| Common Name | 3-Hydroxycycloart-24-en-21-oic acid |
|---|
| Class | Small Molecule |
|---|
| Description | Constituent of Lansium domesticum (langsat). 3-Hydroxycycloart-24-en-21-oic acid is found in fruits. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
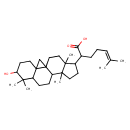
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3-Hydroxycycloart-24-en-21-Oate | Generator | | 2-{6-hydroxy-7,7,12,16-tetramethylpentacyclo[9.7.0.0¹,³.0³,⁸.0¹²,¹⁶]octadecan-15-yl}-6-methylhept-5-enoate | Generator |
|
|---|
| Chemical Formula | C30H48O3 |
|---|
| Average Molecular Mass | 456.700 g/mol |
|---|
| Monoisotopic Mass | 456.360 g/mol |
|---|
| CAS Registry Number | 125302-31-0 |
|---|
| IUPAC Name | 2-{6-hydroxy-7,7,12,16-tetramethylpentacyclo[9.7.0.0¹,³.0³,⁸.0¹²,¹⁶]octadecan-15-yl}-6-methylhept-5-enoic acid |
|---|
| Traditional Name | 2-{6-hydroxy-7,7,12,16-tetramethylpentacyclo[9.7.0.0¹,³.0³,⁸.0¹²,¹⁶]octadecan-15-yl}-6-methylhept-5-enoic acid |
|---|
| SMILES | CC(C)=CCCC(C1CCC2(C)C3CCC4C5(CC35CCC12C)CCC(O)C4(C)C)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C30H48O3/c1-19(2)8-7-9-20(25(32)33)21-12-14-28(6)23-11-10-22-26(3,4)24(31)13-15-29(22)18-30(23,29)17-16-27(21,28)5/h8,20-24,31H,7,9-18H2,1-6H3,(H,32,33) |
|---|
| InChI Key | ATCKKDRMJPISRM-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as cycloartanols and derivatives. These are steroids containing a cycloartanol moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Cycloartanols and derivatives |
|---|
| Direct Parent | Cycloartanols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cycloartanol-skeleton
- 9b,19-cyclo-lanostane-skeleton
- Cycloartane-skeleton
- Triterpenoid
- Hydroxy bile acid, alcohol, or derivatives
- Monohydroxy bile acid, alcohol, or derivatives
- Bile acid, alcohol, or derivatives
- Steroid acid
- 3-hydroxysteroid
- Hydroxysteroid
- Medium-chain fatty acid
- Hydroxy fatty acid
- Fatty acyl
- Fatty acid
- Unsaturated fatty acid
- Cyclic alcohol
- Secondary alcohol
- Carboxylic acid
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Organooxygen compound
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00mo-4042900000-a19ca05ad060169bee3b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-000i-4002190000-c84aa81532186826e00d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052r-0002900000-824aceaa356c11f87b1e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-02hl-3019800000-80aea017b39415b84163 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05mn-4049100000-4f093b4ca9df892208ad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0001900000-063b71bdc90e166722ae | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-06r6-1104900000-05e3a543018f9f637836 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-4129300000-844ae54c11b74b6eea15 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0000900000-6ed6b037889cef897e7b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-08fr-0003900000-24e5d6f9014a78b26f6f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-074j-8209500000-d1cd5d33dabbba866dff | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-2017900000-61f70f64c73a2dd20a76 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0005-9111000000-f4858a01d480308b02be | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00ke-9001000000-df54ff05eb3748d02999 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0038177 |
|---|
| FooDB ID | FDB017416 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 14486857 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|