| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:44:24 UTC |
|---|
| Update Date | 2016-11-09 01:19:17 UTC |
|---|
| Accession Number | CHEM031554 |
|---|
| Identification |
|---|
| Common Name | Isolimonic acid |
|---|
| Class | Small Molecule |
|---|
| Description | Isolimonic acid is found in citrus. Isolimonic acid is a constituent of Citrus species. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
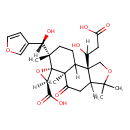
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Isolimonate | Generator | | (3's,5AR,6R,7S,9BR)-9b-(2-carboxy-1-hydroxyethyl)-7-[(R)-(furan-3-yl)(hydroxy)methyl]-3,3,5a,7-tetramethyl-5-oxo-decahydro-1H-spiro[naphtho[1,2-c]furan-6,2'-oxirane]-3'-carboxylate | HMDB |
|
|---|
| Chemical Formula | C26H34O10 |
|---|
| Average Molecular Mass | 506.542 g/mol |
|---|
| Monoisotopic Mass | 506.215 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (3'S,5aR,6R,7S,9bR)-9b-(2-carboxy-1-hydroxyethyl)-7-[(R)-furan-3-yl(hydroxy)methyl]-3,3,5a,7-tetramethyl-5-oxo-decahydro-1H-spiro[naphtho[1,2-c]furan-6,2'-oxirane]-3'-carboxylic acid |
|---|
| Traditional Name | (3'S,5aR,6R,7S,9bR)-9b-(2-carboxy-1-hydroxyethyl)-7-[(R)-furan-3-yl(hydroxy)methyl]-3,3,5a,7-tetramethyl-5-oxo-hexahydrospiro[naphtho[1,2-c]furan-6,2'-oxirane]-3'-carboxylic acid |
|---|
| SMILES | CC1(C)OC[C@]2(C(O)CC(O)=O)C1CC(=O)[C@]1(C)C2CC[C@@](C)([C@@H](O)C2=COC=C2)[C@@]11O[C@@H]1C(O)=O |
|---|
| InChI Identifier | InChI=1S/C26H34O10/c1-22(2)15-9-16(27)24(4)14(25(15,12-35-22)17(28)10-18(29)30)5-7-23(3,19(31)13-6-8-34-11-13)26(24)20(36-26)21(32)33/h6,8,11,14-15,17,19-20,28,31H,5,7,9-10,12H2,1-4H3,(H,29,30)(H,32,33)/t14?,15?,17?,19-,20+,23-,24-,25+,26+/m0/s1 |
|---|
| InChI Key | JSDNZHXBKWCZDG-RWQHKGFASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as naphthofurans. Naphthofurans are compounds containing a furan ring fused to a naphthalene moiety. Furan is a 5 membered- ring aromatic ring with four carbon and one oxygen atoms. Naphthalene is a polycyclic aromatic hydrocarbon made up of two fused benzene rings. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Naphthofurans |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Naphthofurans |
|---|
| Alternative Parents | |
|---|
| Substituents | - Naphthofuran
- Carbocyclic fatty acid
- Beta-hydroxy acid
- Hydroxy fatty acid
- Dicarboxylic acid or derivatives
- Fatty acyl
- Hydroxy acid
- Oxirane carboxylic acid or derivatives
- Oxirane carboxylic acid
- Heteroaromatic compound
- Furan
- Tetrahydrofuran
- Secondary alcohol
- Ketone
- Carboxylic acid derivative
- Carboxylic acid
- Dialkyl ether
- Oxirane
- Ether
- Oxacycle
- Organic oxygen compound
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Alcohol
- Aromatic alcohol
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-9141700000-092cc167e5d15e69f1c4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-002k-9011033000-3fc04633d52f55108a51 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0079-0000910000-2035b8fc46f2e1bf11e6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00dj-1000900000-ef37fbf7710d571b3c71 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-006t-9301600000-d6693411858fccc68d27 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0bt9-2001930000-7080be3a00387f36474c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-066r-7003900000-3820e530ba61f2c43311 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-6009600000-6995e1edf6133fd68f3f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4r-0000790000-205e2112673c894cd08e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4r-0000920000-48b50baf77cbd10b3407 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0092-4455900000-4cac8f8a8ae048e8a660 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-02t9-0000900000-c43d893ec123b082d483 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0005900000-494588f2be395dd8ff9b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00r6-9001200000-020f8fd3d1ae87b74483 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0038170 |
|---|
| FooDB ID | FDB017401 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35014526 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131752314 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|