| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:43:15 UTC |
|---|
| Update Date | 2016-11-09 01:19:17 UTC |
|---|
| Accession Number | CHEM031530 |
|---|
| Identification |
|---|
| Common Name | Calamin |
|---|
| Class | Small Molecule |
|---|
| Description | 2,5-Diethyl-3-methylpyrazine is a flavouring ingredient. It is found in various cooked foods, e.g. potato, beef, pork, cocoa, coffee, bread, sesame seed and soyabean. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
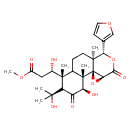
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3,6-Diethyl-2-methylpyrazine | HMDB | | FEMA 3915 | HMDB | | Onguent de calamine | HMDB | | Methyl (3S)-3-[(1R,2S,3R,6R,10S,11R,14S)-11-(furan-3-yl)-3-hydroxy-5-(2-hydroxypropan-2-yl)-2,6,10-trimethyl-4,13-dioxo-12,15-dioxatetracyclo[8.5.0.0¹,¹⁴.0²,⁷]pentadecan-6-yl]-3-hydroxypropanoic acid | Generator |
|
|---|
| Chemical Formula | C27H36O10 |
|---|
| Average Molecular Mass | 520.569 g/mol |
|---|
| Monoisotopic Mass | 520.231 g/mol |
|---|
| CAS Registry Number | 74751-40-9 |
|---|
| IUPAC Name | methyl (3S)-3-[(1R,2S,3R,6R,10S,11R,14S)-11-(furan-3-yl)-3-hydroxy-5-(2-hydroxypropan-2-yl)-2,6,10-trimethyl-4,13-dioxo-12,15-dioxatetracyclo[8.5.0.0¹,¹⁴.0²,⁷]pentadecan-6-yl]-3-hydroxypropanoate |
|---|
| Traditional Name | methyl (3S)-3-[(1R,2S,3R,6R,10S,11R,14S)-11-(furan-3-yl)-3-hydroxy-5-(2-hydroxypropan-2-yl)-2,6,10-trimethyl-4,13-dioxo-12,15-dioxatetracyclo[8.5.0.0¹,¹⁴.0²,⁷]pentadecan-6-yl]-3-hydroxypropanoate |
|---|
| SMILES | COC(=O)C[C@H](O)[C@]1(C)C2CC[C@@]3(C)[C@@H](OC(=O)[C@H]4O[C@@]34[C@]2(C)[C@@H](O)C(=O)C1C(C)(C)O)C1=COC=C1 |
|---|
| InChI Identifier | InChI=1S/C27H36O10/c1-23(2,33)18-17(30)19(31)26(5)14(25(18,4)15(28)11-16(29)34-6)7-9-24(3)20(13-8-10-35-12-13)36-22(32)21-27(24,26)37-21/h8,10,12,14-15,18-21,28,31,33H,7,9,11H2,1-6H3/t14?,15-,18?,19-,20-,21+,24-,25-,26-,27+/m0/s1 |
|---|
| InChI Key | DQSNUOLMAKKASD-KUNOYEQWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyrazines. Pyrazines are compounds containing a pyrazine ring, which is a six-member aromatic heterocycle, that consists of two nitrogen atoms (at positions 1 and 4) and four carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Diazines |
|---|
| Sub Class | Pyrazines |
|---|
| Direct Parent | Pyrazines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyrazine
- Heteroaromatic compound
- Azacycle
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organonitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-06rl-9024520000-8296ef186fedd5235145 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-000t-6251309000-61f8671fe8a5d1af680a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_3_4) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("Calamin,3TBDMS,#4" TMS) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uki-0000950000-afe93ace095a68cb6a98 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f7a-0000920000-ab3ac5ee773b149e3de3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000b-9125710000-27968ae4324fca1a7bd0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00or-1000940000-353bf208ff76502eead3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0g4i-3000930000-dfb1bdc4b67da9294145 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05te-8001900000-70d541406429a98a8d89 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0g4i-0000950000-a09daf8f0b0ba9418d74 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0v6r-1034930000-b8d617102e8aa48c1ad8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-002n-9215300000-ab12b3121f7a0be7d86c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-02t9-0001970000-2db0a928c81baa603ec8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01oy-3003910000-4b1fa0295fe18ec45bf6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-9000000000-58056a82f83bb536ef5a | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036132 |
|---|
| FooDB ID | FDB014981 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 33311 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 36225 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|