| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:42:29 UTC |
|---|
| Update Date | 2016-11-09 01:19:16 UTC |
|---|
| Accession Number | CHEM031513 |
|---|
| Identification |
|---|
| Common Name | 1,7,7-Trimethyltricyclo[2.2.1.02,6]heptane |
|---|
| Class | Small Molecule |
|---|
| Description | A monoterpene that is tricyclo[2.2.1.0(2,6)]heptane bearing a three additional methyl substituents (one at position 1 and two at position 7). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
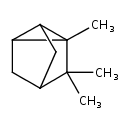
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,1,7-trimethyltricyclo(2.2.1.0(2.6))Heptane | HMDB | | 1,7,7-Trimethyl-tricyclo(2.2.1.02,6)heptane | HMDB | | 1,7,7-Trimethyl-tricyclo[2.2.1.0(2,6)]heptane | HMDB | | 1,7,7-Trimethyl-tricyclo[2.2.1.0*2,6*]heptane | HMDB | | 1,7,7-Trimethyl-tricyclo[2.2.1.02,6]heptane | HMDB | | 1,7,7-trimethyltricyclo(2.2.1.02,6)Heptane | HMDB | | 1,7,7-trimethyltricyclo[2.2.1.0,2,6]Heptane | HMDB | | alpha-Tricyclene | HMDB | | Cyclene | HMDB | | Tricyclene | HMDB | | 1,7,7-Trimethyltricyclo[2.2.1.02,6]heptane | PhytoBank | | Teresantanane | PhytoBank | | Tricyclane | PhytoBank |
|
|---|
| Chemical Formula | C10H16 |
|---|
| Average Molecular Mass | 136.238 g/mol |
|---|
| Monoisotopic Mass | 136.125 g/mol |
|---|
| CAS Registry Number | 508-32-7 |
|---|
| IUPAC Name | 1,7,7-trimethyltricyclo[2.2.1.0^{2,6}]heptane |
|---|
| Traditional Name | 1,7,7-trimethyltricyclo[2.2.1.0^{2,6}]heptane |
|---|
| SMILES | CC12C3CC(CC13)C2(C)C |
|---|
| InChI Identifier | InChI=1S/C10H16/c1-9(2)6-4-7-8(5-6)10(7,9)3/h6-8H,4-5H2,1-3H3 |
|---|
| InChI Key | RRBYUSWBLVXTQN-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as bicyclic monoterpenoids. These are monoterpenoids containing exactly 2 rings, which are fused to each other. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Monoterpenoids |
|---|
| Direct Parent | Bicyclic monoterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Bornane monoterpenoid
- Polycyclic hydrocarbon
- Saturated hydrocarbon
- Hydrocarbon
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00dr-1900000000-0381445b90995eb87ca9 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0900000000-89e84f859d8f1f97d5c2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0900000000-fb8d56b761aae12a505c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00dr-0900000000-bfe7e4ba927cd83956b4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0900000000-5e150dd4370565464dad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0900000000-5e150dd4370565464dad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kr-0900000000-b5dcbaea24fd03f1cbf9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0900000000-32bfa268614bcd6c4f81 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0900000000-9aed2aabe2ae62519804 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-0900000000-d49f9a597cdeb89b1abf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0900000000-a013b4ae27f975ab5621 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0900000000-a013b4ae27f975ab5621 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-0900000000-afdd20de568fd3df81ea | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0038121 |
|---|
| FooDB ID | FDB017346 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00011067 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | CPD-4902 |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 71367 |
|---|
| ChEBI ID | 64266 |
|---|
| PubChem Compound ID | 79035 |
|---|
| Kegg Compound ID | C20241 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|