| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:40:34 UTC |
|---|
| Update Date | 2016-11-09 01:19:16 UTC |
|---|
| Accession Number | CHEM031470 |
|---|
| Identification |
|---|
| Common Name | 1,28-Octacosanediol diferulate |
|---|
| Class | Small Molecule |
|---|
| Description | 1,28-Octacosanediol diferulate is found in cereals and cereal products. 1,28-Octacosanediol diferulate is found in oats (Avena sativa). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
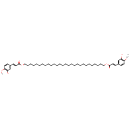
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,28-Octacosanediol diferulic acid | Generator | | 28-{[(2E)-3-(3-hydroxy-4-methoxyphenyl)prop-2-enoyl]oxy}octacosyl (2E)-3-(3-hydroxy-4-methoxyphenyl)prop-2-enoic acid | HMDB |
|
|---|
| Chemical Formula | C48H74O8 |
|---|
| Average Molecular Mass | 779.096 g/mol |
|---|
| Monoisotopic Mass | 778.538 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 28-{[(2E)-3-(3-hydroxy-4-methoxyphenyl)prop-2-enoyl]oxy}octacosyl (2E)-3-(3-hydroxy-4-methoxyphenyl)prop-2-enoate |
|---|
| Traditional Name | 28-{[(2E)-3-(3-hydroxy-4-methoxyphenyl)prop-2-enoyl]oxy}octacosyl (2E)-3-(3-hydroxy-4-methoxyphenyl)prop-2-enoate |
|---|
| SMILES | COC1=C(O)C=C(\C=C\C(=O)OCCCCCCCCCCCCCCCCCCCCCCCCCCCCOC(=O)\C=C\C2=CC(O)=C(OC)C=C2)C=C1 |
|---|
| InChI Identifier | InChI=1S/C48H74O8/c1-53-45-33-29-41(39-43(45)49)31-35-47(51)55-37-27-25-23-21-19-17-15-13-11-9-7-5-3-4-6-8-10-12-14-16-18-20-22-24-26-28-38-56-48(52)36-32-42-30-34-46(54-2)44(50)40-42/h29-36,39-40,49-50H,3-28,37-38H2,1-2H3/b35-31+,36-32+ |
|---|
| InChI Key | BNCYSRWQCKLMQU-QUTRQNJUSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as coumaric acids and derivatives. These are aromatic compounds containing Aromatic compounds containing a cinnamic acid moiety (or a derivative thereof) hydroxylated at the C2 (ortho-), C3 (meta-), or C4 (para-) carbon atom of the benzene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Cinnamic acids and derivatives |
|---|
| Sub Class | Hydroxycinnamic acids and derivatives |
|---|
| Direct Parent | Coumaric acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Coumaric acid or derivatives
- Fatty alcohol ester
- Cinnamic acid ester
- Methoxyphenol
- Anisole
- Phenoxy compound
- Phenol ether
- Styrene
- Methoxybenzene
- Alkyl aryl ether
- Fatty acid ester
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Fatty acyl
- Dicarboxylic acid or derivatives
- Benzenoid
- Monocyclic benzene moiety
- Enoate ester
- Alpha,beta-unsaturated carboxylic ester
- Carboxylic acid ester
- Ether
- Carboxylic acid derivative
- Organooxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004j-1964020000-99d64a3e9fd089e5dcec | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0400021900-1649e9f04a9c5b7672a9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0914242200-c956faf72cc1d538b551 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0adj-0658970000-c5c5de616c5c12246593 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0900011800-dda11a0ce022d2b293b1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004l-0900000100-4892e8f92dba416b909b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004l-0900000000-bdf7b6c48d281b6807f2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0700010900-2aac359625e0fa36e82e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fc0-0910174600-58200ba3776fe8dcb921 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01tj-0902621000-b2cf987696853e271ad9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004j-0900001800-960ddf11406fc338c88e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001j-0900001100-db2d409e7d4bd323f4b1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000t-0900000000-3186314bba8af047ccf6 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0038065 |
|---|
| FooDB ID | FDB017277 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 30777228 |
|---|
| ChEBI ID | 176317 |
|---|
| PubChem Compound ID | 131752291 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|