| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:33:36 UTC |
|---|
| Update Date | 2016-11-09 01:19:14 UTC |
|---|
| Accession Number | CHEM031315 |
|---|
| Identification |
|---|
| Common Name | 2-Butyl-5-methyl-4-propyloxazole |
|---|
| Class | Small Molecule |
|---|
| Description | 2-Butyl-5-methyl-4-propyloxazole is found in potato. 2-Butyl-5-methyl-4-propyloxazole is a constituent of French fries. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
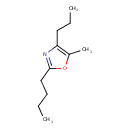
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C11H19NO |
|---|
| Average Molecular Mass | 181.275 g/mol |
|---|
| Monoisotopic Mass | 181.147 g/mol |
|---|
| CAS Registry Number | 94794-09-9 |
|---|
| IUPAC Name | 2-butyl-5-methyl-4-propyl-1,3-oxazole |
|---|
| Traditional Name | 2-butyl-5-methyl-4-propyl-1,3-oxazole |
|---|
| SMILES | CCCCC1=NC(CCC)=C(C)O1 |
|---|
| InChI Identifier | InChI=1S/C11H19NO/c1-4-6-8-11-12-10(7-5-2)9(3)13-11/h4-8H2,1-3H3 |
|---|
| InChI Key | JIJACJIEHHDTAK-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 2,4,5-trisubstituted oxazoles. 2,4,5-trisubstituted oxazoles are compounds containing an oxazole ring substituted at positions 2, 4 and 5 only. Oxazole is a five-membered aromatic heterocycle with one oxygen, one nitrogen, and three carbon atoms. Isomers include 1,2-oxazole and 1,3-oxazole. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Azoles |
|---|
| Sub Class | Oxazoles |
|---|
| Direct Parent | 2,4,5-trisubstituted oxazoles |
|---|
| Alternative Parents | |
|---|
| Substituents | - 2,4,5-trisubstituted 1,3-oxazole
- Heteroaromatic compound
- Oxacycle
- Azacycle
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0fvu-6900000000-6f1049b4913f15f98f37 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0900000000-ad25286b0f315b2eeb63 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-6900000000-3f0ab387716450723de8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0k96-9000000000-3b231cc2d1470fef8130 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-3900000000-016693cfc55dc3d6bcdb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001r-2900000000-79302a949c2f633bbc64 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-9200000000-21149d4be5d65e202a9e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0900000000-463dd04e6e84f6c7c33e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-1900000000-6201516524f3e1600659 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-9700000000-53b630ccf40e0f5f772c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0900000000-016b2d7221680eab0632 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001s-4900000000-977706c3558c4934751e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ac3-9200000000-50405abbe34bdb3ae2e2 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0037894 |
|---|
| FooDB ID | FDB017050 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 458439 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 525779 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|