| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:32:48 UTC |
|---|
| Update Date | 2016-11-09 01:19:14 UTC |
|---|
| Accession Number | CHEM031295 |
|---|
| Identification |
|---|
| Common Name | 2-Ethyl-4-methyl-5-propyloxazole |
|---|
| Class | Small Molecule |
|---|
| Description | 2-Ethyl-4-methyl-5-propyloxazole is found in potato. 2-Ethyl-4-methyl-5-propyloxazole is a constituent of French fries. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
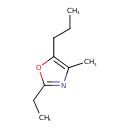
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C9H15NO |
|---|
| Average Molecular Mass | 153.222 g/mol |
|---|
| Monoisotopic Mass | 153.115 g/mol |
|---|
| CAS Registry Number | 102586-53-8 |
|---|
| IUPAC Name | 2-ethyl-4-methyl-5-propyl-1,3-oxazole |
|---|
| Traditional Name | 2-ethyl-4-methyl-5-propyl-1,3-oxazole |
|---|
| SMILES | CCCC1=C(C)N=C(CC)O1 |
|---|
| InChI Identifier | InChI=1S/C9H15NO/c1-4-6-8-7(3)10-9(5-2)11-8/h4-6H2,1-3H3 |
|---|
| InChI Key | FDMDJAGBUGUESB-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 2,4,5-trisubstituted oxazoles. 2,4,5-trisubstituted oxazoles are compounds containing an oxazole ring substituted at positions 2, 4 and 5 only. Oxazole is a five-membered aromatic heterocycle with one oxygen, one nitrogen, and three carbon atoms. Isomers include 1,2-oxazole and 1,3-oxazole. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Azoles |
|---|
| Sub Class | Oxazoles |
|---|
| Direct Parent | 2,4,5-trisubstituted oxazoles |
|---|
| Alternative Parents | |
|---|
| Substituents | - 2,4,5-trisubstituted 1,3-oxazole
- Heteroaromatic compound
- Oxacycle
- Azacycle
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00bi-7900000000-3fe655d8c36c32fd2e95 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0900000000-81623969cc569a7e33e5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-7900000000-12b794d1b220d83e6452 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ugl-9000000000-a1877d4cc0347d99bc8f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-1900000000-9b200cc2b706ec6e9a73 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0f89-9500000000-95309baad05f06aefe43 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9200000000-fe32bc6002afcb8f49bb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0900000000-8697d208f7c47827d4cf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-2900000000-3f532b07bd614d2a27ca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0f6x-9200000000-3958e89439e28fbd0a5e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0900000000-3ab752e8b93070467cee | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-6900000000-b199e0011ab318de75c5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a5c-9100000000-18a11f95b292aa199bc6 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0037872 |
|---|
| FooDB ID | FDB017025 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 458440 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 525780 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|