| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:30:28 UTC |
|---|
| Update Date | 2016-11-09 01:19:13 UTC |
|---|
| Accession Number | CHEM031247 |
|---|
| Identification |
|---|
| Common Name | Annuolide A |
|---|
| Class | Small Molecule |
|---|
| Description | Annuolide A is found in fats and oils. Annuolide A is a constituent of Helianthus annuus (sunflower). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
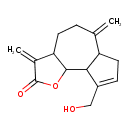
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 15-Hydroxy-3,10(14),11(13)-guaiatrien-12,6-olide | HMDB |
|
|---|
| Chemical Formula | C15H18O3 |
|---|
| Average Molecular Mass | 246.302 g/mol |
|---|
| Monoisotopic Mass | 246.126 g/mol |
|---|
| CAS Registry Number | 152442-48-3 |
|---|
| IUPAC Name | 9-(hydroxymethyl)-3,6-dimethylidene-2H,3H,3aH,4H,5H,6H,6aH,7H,9aH,9bH-azuleno[4,5-b]furan-2-one |
|---|
| Traditional Name | 9-(hydroxymethyl)-3,6-dimethylidene-3aH,4H,5H,6aH,7H,9aH,9bH-azuleno[4,5-b]furan-2-one |
|---|
| SMILES | OCC1=CCC2C1C1OC(=O)C(=C)C1CCC2=C |
|---|
| InChI Identifier | InChI=1S/C15H18O3/c1-8-3-5-12-9(2)15(17)18-14(12)13-10(7-16)4-6-11(8)13/h4,11-14,16H,1-3,5-7H2 |
|---|
| InChI Key | XIKLPPAERLRBPO-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as guaianolides and derivatives. These are diterpene lactones with a structure characterized by the presence of a gamma-lactone fused to a guaiane, forming 3,6,9-trimethyl-azuleno[4,5-b]furan-2-one or a derivative. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Terpene lactones |
|---|
| Direct Parent | Guaianolides and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Guaianolide-skeleton
- Guaiane sesquiterpenoid
- Sesquiterpenoid
- Gamma butyrolactone
- Tetrahydrofuran
- Enoate ester
- Alpha,beta-unsaturated carboxylic ester
- Lactone
- Carboxylic acid ester
- Oxacycle
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organoheterocyclic compound
- Alcohol
- Primary alcohol
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0f6t-4940000000-6599e925fe9f46797b54 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0ukc-3690000000-26fe5fb7227930160080 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004j-0290000000-4264be28ad163a20a252 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-056r-0950000000-2a062bd9c045f7e5f35b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pb9-6910000000-fa02815e794a71d760db | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-ccab6068ec3a6d300b2a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ftb-0290000000-ad38987e4f12417f9144 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0uka-6910000000-76695d46dc80b81b371a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014j-0090000000-41f91938a2388dec7713 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0090000000-99ef8bcde447b8bb37ef | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00y0-0930000000-426d080f825b0133c8bd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0090000000-a3cf7eee8166bfde606a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-002b-0390000000-c9b58398ff95ab25afb3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-06fv-1910000000-c87a76a61e64df7b585d | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0037801 |
|---|
| FooDB ID | FDB016945 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00054894 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35014475 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 75051793 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|