| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:30:02 UTC |
|---|
| Update Date | 2016-11-09 01:19:13 UTC |
|---|
| Accession Number | CHEM031236 |
|---|
| Identification |
|---|
| Common Name | Crassostrea Secocarotenoid |
|---|
| Class | Small Molecule |
|---|
| Description | Crassostrea Secocarotenoid is found in mollusks. Crassostrea Secocarotenoid is a constituent of the oyster Crassostrea gigas. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
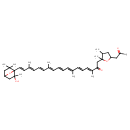
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C40H56O5 |
|---|
| Average Molecular Mass | 616.870 g/mol |
|---|
| Monoisotopic Mass | 616.413 g/mol |
|---|
| CAS Registry Number | 256505-51-8 |
|---|
| IUPAC Name | (3E,5E,7E,9E,11E,13E,15E,17E)-1-[2,3-dimethyl-5-(2-oxopropyl)oxolan-2-yl]-18-{2-hydroxy-2,6,6-trimethyl-7-oxabicyclo[2.2.1]heptan-1-yl}-3,7,12,16-tetramethyloctadeca-3,5,7,9,11,13,15,17-octaen-2-one |
|---|
| Traditional Name | (3E,5E,7E,9E,11E,13E,15E,17E)-1-[2,3-dimethyl-5-(2-oxopropyl)oxolan-2-yl]-18-{2-hydroxy-2,6,6-trimethyl-7-oxabicyclo[2.2.1]heptan-1-yl}-3,7,12,16-tetramethyloctadeca-3,5,7,9,11,13,15,17-octaen-2-one |
|---|
| SMILES | CC1CC(CC(C)=O)OC1(C)CC(=O)C(\C)=C\C=C\C(\C)=C\C=C\C=C(/C)\C=C\C=C(/C)\C=C\C12OC(CC1(C)C)CC2(C)O |
|---|
| InChI Identifier | InChI=1S/C40H56O5/c1-28(17-13-18-30(3)21-22-40-37(7,8)25-35(45-40)26-39(40,10)43)15-11-12-16-29(2)19-14-20-31(4)36(42)27-38(9)32(5)23-34(44-38)24-33(6)41/h11-22,32,34-35,43H,23-27H2,1-10H3/b12-11+,17-13+,19-14+,22-21+,28-15+,29-16+,30-18+,31-20+ |
|---|
| InChI Key | YCHOPPKXFCUQHM-OMSIYMKDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as triterpenoids. These are terpene molecules containing six isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Triterpenoids |
|---|
| Direct Parent | Triterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Triterpenoid
- Monosaccharide
- Alpha-branched alpha,beta-unsaturated-ketone
- Acryloyl-group
- Cyclic alcohol
- Enone
- Alpha,beta-unsaturated ketone
- Tetrahydrofuran
- Tertiary alcohol
- Ketone
- Dialkyl ether
- Ether
- Oxacycle
- Organoheterocyclic compound
- Hydrocarbon derivative
- Organic oxygen compound
- Organic oxide
- Alcohol
- Carbonyl group
- Organooxygen compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0pbc-8500295000-5e260585dfc4b983757e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00dl-8300049000-54ade77cdb2739fb626f | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_4) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("Crassostrea Secocarotenoid,1TMS,#1" TMS) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0121952000-8bdd82670a0bfe231f89 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-002b-1294840000-07014740539fccd2e713 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-2391400000-c6bda0ecb63ace9a90f7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-1300449000-231a2e09564b392d039b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-066r-8800795000-1ebce3cb5ceeac418b2f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9800240000-784760934eb35d3bef28 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0110039000-02354d86e7b16d9f92d3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0aor-5901311000-26d569e8370eea5758a0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-054p-9213271000-04fcfd0e18457a8b3333 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-02ta-0914654000-4f933160e3a6dedf01aa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05o1-2914682000-10393f49497ac501c644 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00lv-1913100000-d274fafbe927c4f8d680 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0037789 |
|---|
| FooDB ID | FDB016933 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00057293 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35014469 |
|---|
| ChEBI ID | 176236 |
|---|
| PubChem Compound ID | 131752233 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|