| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:28:55 UTC |
|---|
| Update Date | 2016-11-09 01:19:13 UTC |
|---|
| Accession Number | CHEM031210 |
|---|
| Identification |
|---|
| Common Name | Hexamethylgossypetin |
|---|
| Class | Small Molecule |
|---|
| Description | 3,3',4',5,7,8-Hexamethoxyflavone is found in citrus. 3,3',4',5,7,8-Hexamethoxyflavone is isolated from Valencia orange peel (Citrus sinensis) and shepherds purse (Capsella bursa-pastoris). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
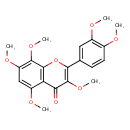
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-(3,4-Dimethoxyphenyl)-3,5,7,8-tetramethoxy-4H-1-benzopyran-4-one | HMDB | | 3,5,7,8,3',4'-Hexamethoxyflavone | HMDB | | 3-chloro-4-Methylanilinium hydrogen sulphate | HMDB | | Gossypetin hexamethyl ether | HMDB |
|
|---|
| Chemical Formula | C21H22O8 |
|---|
| Average Molecular Mass | 402.395 g/mol |
|---|
| Monoisotopic Mass | 402.131 g/mol |
|---|
| CAS Registry Number | 7741-47-1 |
|---|
| IUPAC Name | 2-(3,4-dimethoxyphenyl)-3,5,7,8-tetramethoxy-4H-chromen-4-one |
|---|
| Traditional Name | gossypetin hexamethyl ether |
|---|
| SMILES | COC1=CC(OC)=C(OC)C2=C1C(=O)C(OC)=C(O2)C1=CC(OC)=C(OC)C=C1 |
|---|
| InChI Identifier | InChI=1S/C21H22O8/c1-23-12-8-7-11(9-13(12)24-2)18-21(28-6)17(22)16-14(25-3)10-15(26-4)19(27-5)20(16)29-18/h7-10H,1-6H3 |
|---|
| InChI Key | XBZIUXVIWRAJKB-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 8-o-methylated flavonoids. These are flavonoids with methoxy groups attached to the C8 atom of the flavonoid backbone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Flavonoids |
|---|
| Sub Class | O-methylated flavonoids |
|---|
| Direct Parent | 8-O-methylated flavonoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - 3p-methoxyflavonoid-skeleton
- 3-methoxyflavonoid-skeleton
- 4p-methoxyflavonoid-skeleton
- 5-methoxyflavonoid-skeleton
- 7-methoxyflavonoid-skeleton
- 8-methoxyflavonoid-skeleton
- Flavone
- 3-methoxychromone
- Chromone
- 1-benzopyran
- Benzopyran
- O-dimethoxybenzene
- Dimethoxybenzene
- Phenoxy compound
- Anisole
- Methoxybenzene
- Phenol ether
- Alkyl aryl ether
- Pyranone
- Pyran
- Benzenoid
- Monocyclic benzene moiety
- Vinylogous ester
- Heteroaromatic compound
- Ether
- Organoheterocyclic compound
- Oxacycle
- Organic oxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00dr-0219000000-5ffc5aa8b873cf9ae692 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0000900000-56d67cbecf78fc5d3f10 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0001900000-932172e09c721220bb8e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-045i-1359000000-e34c767d835e69e2f0fd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0000900000-96ecc0f5180a95facdec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0006900000-94619482929b580cd219 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-08g3-1739000000-78d10512872fe28d61bd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0000900000-8d74c9163b0fa9cff6fe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0031900000-a911f7ec7597f07b81af | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4s-1932100000-43e437324dc46f9a08a3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0000900000-3ecd8c89d3cf77141250 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0000900000-5e387adacb734b7a51ed | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ik9-2191300000-8e53a6fe976d38ebc797 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0037755 |
|---|
| FooDB ID | FDB016894 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00004745 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 128872 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 146093 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|