| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:24:35 UTC |
|---|
| Update Date | 2016-11-09 01:19:12 UTC |
|---|
| Accession Number | CHEM031125 |
|---|
| Identification |
|---|
| Common Name | 10beta-12,13-Dinor-8-oxo-6-eremophilen-11-al |
|---|
| Class | Small Molecule |
|---|
| Description | 10beta-12,13-Dinor-8-oxo-6-eremophilen-11-al is found in green vegetables. 10beta-12,13-Dinor-8-oxo-6-eremophilen-11-al is a constituent of Petasites japonicus ssp. giganteus (Japanese butterbur). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
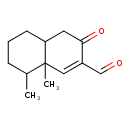
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 10b-12,13-Dinor-8-oxo-6-eremophilen-11-al | Generator | | 10Β-12,13-dinor-8-oxo-6-eremophilen-11-al | Generator |
|
|---|
| Chemical Formula | C13H18O2 |
|---|
| Average Molecular Mass | 206.281 g/mol |
|---|
| Monoisotopic Mass | 206.131 g/mol |
|---|
| CAS Registry Number | 348119-86-8 |
|---|
| IUPAC Name | 8,8a-dimethyl-3-oxo-3,4,4a,5,6,7,8,8a-octahydronaphthalene-2-carbaldehyde |
|---|
| Traditional Name | 8,8a-dimethyl-3-oxo-4,4a,5,6,7,8-hexahydronaphthalene-2-carbaldehyde |
|---|
| SMILES | CC1CCCC2CC(=O)C(C=O)=CC12C |
|---|
| InChI Identifier | InChI=1S/C13H18O2/c1-9-4-3-5-11-6-12(15)10(8-14)7-13(9,11)2/h7-9,11H,3-6H2,1-2H3 |
|---|
| InChI Key | QJHOMNLQPDCBJF-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as cyclohexenones. Cyclohexenones are compounds containing a cylohexenone moiety, which is a six-membered aliphatic ring that carries a ketone and has one endocyclic double bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbonyl compounds |
|---|
| Direct Parent | Cyclohexenones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cyclohexenone
- Organic oxide
- Hydrocarbon derivative
- Aldehyde
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01r6-0900000000-ec27beb798b1094adedb | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0590000000-97bbb8494cfa845ddcbd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4r-2920000000-debed636ea568702f5ba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014l-9700000000-12b688dddc3af1641bd2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0190000000-86bde4a9e533a56c2a6f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0290000000-f12c300feb2118126337 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000f-5900000000-4426802c2fdc22be9dbd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0090000000-5535f56d1e770e37902d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0190000000-c1f703aeace4f458060d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00ds-0900000000-3a0d2e5e74ea14cb9ab2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0390000000-997f078ed503ecd6924a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-2900000000-3cbef304a3324e2d361d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052o-6900000000-fef2b1b2359cebf5959f | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0037604 |
|---|
| FooDB ID | FDB016717 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35014442 |
|---|
| ChEBI ID | 138757 |
|---|
| PubChem Compound ID | 85239007 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|