| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:19:56 UTC |
|---|
| Update Date | 2016-11-09 01:19:11 UTC |
|---|
| Accession Number | CHEM031021 |
|---|
| Identification |
|---|
| Common Name | Folerogenin |
|---|
| Class | Small Molecule |
|---|
| Description | Folerogenin is found in root vegetables. Folerogenin is isolated from licorice (Glycyrrhiza glabra) leaves Nadolol is a nonselective beta-adrenergic receptor antagonist with a long half-life, and is structurally similar to propranolol. Clinical pharmacology studies have demonstrated beta-blocking activity by showing (1) reduction in heart rate and cardiac output at rest and on exercise, (2) reduction of systolic and diastolic blood pressure at rest and on exercise, (3) inhibition of isoproterenol-induced tachycardia, and (4) reduction of reflex orthostatic tachycardia. Nadolol has no intrinsic sympathomimetic activity and, unlike some other beta-adrenergic blocking agents, nadolol has little direct myocardial depressant activity and does not have an anesthetic-like membrane-stabilizing action. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
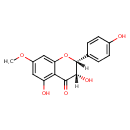
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Anabet | HMDB | | Corgard | HMDB | | Corgaretic | HMDB | | Corzide | HMDB | | Isoauroside | HMDB | | Nadic | HMDB | | Nadolol | HMDB | | Nadolol (JP15/usp/inn) | HMDB | | Nadololum | HMDB | | Solgol | HMDB |
|
|---|
| Chemical Formula | C16H14O6 |
|---|
| Average Molecular Mass | 302.279 g/mol |
|---|
| Monoisotopic Mass | 302.079 g/mol |
|---|
| CAS Registry Number | 35815-06-6 |
|---|
| IUPAC Name | (2R,3S)-3,5-dihydroxy-2-(4-hydroxyphenyl)-7-methoxy-3,4-dihydro-2H-1-benzopyran-4-one |
|---|
| Traditional Name | (2R,3S)-3,5-dihydroxy-2-(4-hydroxyphenyl)-7-methoxy-2,3-dihydro-1-benzopyran-4-one |
|---|
| SMILES | COC1=CC(O)=C2C(=O)[C@@H](O)[C@H](OC2=C1)C1=CC=C(O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C16H14O6/c1-21-10-6-11(18)13-12(7-10)22-16(15(20)14(13)19)8-2-4-9(17)5-3-8/h2-7,15-18,20H,1H3/t15-,16-/m1/s1 |
|---|
| InChI Key | LZLGHWHSUZVUFZ-HZPDHXFCSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 7-o-methylated flavonoids. These are flavonoids with methoxy groups attached to the C7 atom of the flavonoid backbone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Flavonoids |
|---|
| Sub Class | O-methylated flavonoids |
|---|
| Direct Parent | 7-O-methylated flavonoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - 7-methoxyflavonoid-skeleton
- 3-hydroxyflavonoid
- 4'-hydroxyflavonoid
- 5-hydroxyflavonoid
- Flavanone
- Flavanonol
- Hydroxyflavonoid
- Flavan
- Chromone
- Chromane
- Benzopyran
- 1-benzopyran
- Anisole
- Aryl alkyl ketone
- Aryl ketone
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Alkyl aryl ether
- 1-hydroxy-4-unsubstituted benzenoid
- Benzenoid
- Monocyclic benzene moiety
- Vinylogous acid
- Ketone
- Secondary alcohol
- Ether
- Organoheterocyclic compound
- Oxacycle
- Organic oxygen compound
- Alcohol
- Organic oxide
- Organooxygen compound
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0ab9-0950000000-de2029bc14e7db044c3b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0uka-9820550000-5b30b68052967e7e8cb0 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0139000000-3d001fea9798a00fa93a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0v4i-0943000000-9058f0c38fbb5883ca55 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-3900000000-c31b22a759a19c82ea1c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0219000000-0b14661f38b7633fbbee | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0v4r-1944000000-182176261c015d80cb5b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00rm-5910000000-ab9ec644ac85d4ed0128 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0409000000-a2ab31c5228f28769ce4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0900000000-a7ee695a918b69294573 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-0900000000-ff30aa4d3f57671329de | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0037507 |
|---|
| FooDB ID | FDB016583 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00020493 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 30777189 |
|---|
| ChEBI ID | 7444 |
|---|
| PubChem Compound ID | 5319509 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|