| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:18:45 UTC |
|---|
| Update Date | 2016-11-09 01:19:11 UTC |
|---|
| Accession Number | CHEM030994 |
|---|
| Identification |
|---|
| Common Name | 5,7-Dihydroxy-3-(3-hydroxy-4-methoxybenzyl)-4-chromanone |
|---|
| Class | Small Molecule |
|---|
| Description | 5,7-Dihydroxy-3-(3-hydroxy-4-methoxybenzyl)-4-chromanone is found in herbs and spices. 5,7-Dihydroxy-3-(3-hydroxy-4-methoxybenzyl)-4-chromanone is from Muscari comosum (tassel hyacinth). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
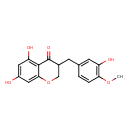
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3'-Hydroxy-3,9-dihydroeucomin | HMDB |
|
|---|
| Chemical Formula | C17H16O6 |
|---|
| Average Molecular Mass | 316.305 g/mol |
|---|
| Monoisotopic Mass | 316.095 g/mol |
|---|
| CAS Registry Number | 107585-75-1 |
|---|
| IUPAC Name | 5,7-dihydroxy-3-[(3-hydroxy-4-methoxyphenyl)methyl]-3,4-dihydro-2H-1-benzopyran-4-one |
|---|
| Traditional Name | 5,7-dihydroxy-3-[(3-hydroxy-4-methoxyphenyl)methyl]-2,3-dihydro-1-benzopyran-4-one |
|---|
| SMILES | COC1=C(O)C=C(CC2COC3=CC(O)=CC(O)=C3C2=O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C17H16O6/c1-22-14-3-2-9(5-12(14)19)4-10-8-23-15-7-11(18)6-13(20)16(15)17(10)21/h2-3,5-7,10,18-20H,4,8H2,1H3 |
|---|
| InChI Key | WIBOONWRYQFYQJ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as homoisoflavanones. These are homoisoflavonoids with a structure based on the chromanone system. Chromanone is a bicyclic compound consisting of a 3,4-dihydro-1-benzopyran, which bears a ketone group at the 4-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Homoisoflavonoids |
|---|
| Sub Class | Homoisoflavans |
|---|
| Direct Parent | Homoisoflavanones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Homoisoflavanone
- Chromone
- Methoxyphenol
- Chromane
- Benzopyran
- 1-benzopyran
- Phenoxy compound
- Anisole
- Phenol ether
- Methoxybenzene
- Aryl alkyl ketone
- Aryl ketone
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Phenol
- Monocyclic benzene moiety
- Benzenoid
- Vinylogous acid
- Ketone
- Oxacycle
- Organoheterocyclic compound
- Ether
- Organic oxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000i-0963000000-2a1edeb0e952652a69c7 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-02t9-3690770000-72b6a793545746ece3f7 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0529000000-87ceb5329045b17d4eb3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uxr-0922000000-eb96fe3c02f7711318e9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udr-2900000000-3f656bc4c9d7f1d5c32e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0219000000-89b2d608155560735295 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0779000000-552904ca86e6820c16ff | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-1950000000-3737a5d2a6cb2056648e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0009000000-7195ac3ed96f1c08ffd1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0904000000-d21483a82a4cebbfd49d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gdl-1920000000-d425754d77f73bf0e1cb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0009000000-a5811c0f9e3fc0f9d174 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-016r-0449000000-8e63a594e26468dd5083 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03di-1943000000-2731db78ee0274f677cf | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0037477 |
|---|
| FooDB ID | FDB016542 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00056425 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 358308 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 404571 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|