| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:13:44 UTC |
|---|
| Update Date | 2016-11-09 01:19:09 UTC |
|---|
| Accession Number | CHEM030870 |
|---|
| Identification |
|---|
| Common Name | Quercetin 3,3'-diglucoside |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
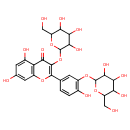
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C27H30O17 |
|---|
| Average Molecular Mass | 626.517 g/mol |
|---|
| Monoisotopic Mass | 626.148 g/mol |
|---|
| CAS Registry Number | 20188-84-5 |
|---|
| IUPAC Name | 5,7-dihydroxy-2-(4-hydroxy-3-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}phenyl)-3-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-4H-chromen-4-one |
|---|
| Traditional Name | 5,7-dihydroxy-2-(4-hydroxy-3-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}phenyl)-3-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}chromen-4-one |
|---|
| SMILES | OCC1OC(OC2=C(O)C=CC(=C2)C2=C(OC3OC(CO)C(O)C(O)C3O)C(=O)C3=C(O2)C=C(O)C=C3O)C(O)C(O)C1O |
|---|
| InChI Identifier | InChI=1S/C27H30O17/c28-6-14-17(33)20(36)22(38)26(42-14)41-12-3-8(1-2-10(12)31)24-25(19(35)16-11(32)4-9(30)5-13(16)40-24)44-27-23(39)21(37)18(34)15(7-29)43-27/h1-5,14-15,17-18,20-23,26-34,36-39H,6-7H2 |
|---|
| InChI Key | VAFSBVRKSHSWHW-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as flavonoid-3-o-glycosides. These are phenolic compounds containing a flavonoid moiety which is O-glycosidically linked to carbohydrate moiety at the C3-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Flavonoids |
|---|
| Sub Class | Flavonoid glycosides |
|---|
| Direct Parent | Flavonoid-3-O-glycosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Flavonoid-3-o-glycoside
- 4'-hydroxyflavonoid
- 5-hydroxyflavonoid
- 7-hydroxyflavonoid
- Flavone
- Hydroxyflavonoid
- Glycosyl compound
- Disaccharide
- O-glycosyl compound
- Chromone
- Benzopyran
- 1-benzopyran
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Pyranone
- Phenol
- Monocyclic benzene moiety
- Benzenoid
- Pyran
- Oxane
- Vinylogous acid
- Heteroaromatic compound
- Secondary alcohol
- Carboxylic acid ester
- Acetal
- Oxacycle
- Organoheterocyclic compound
- Carboxylic acid derivative
- Polyol
- Monocarboxylic acid or derivatives
- Organic oxide
- Hydrocarbon derivative
- Carbonyl group
- Organic oxygen compound
- Alcohol
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05mk-0011904000-57a091db779d86e9b740 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udj-0118900000-5b647586bd024070f032 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-1439400000-805995eed481f5189829 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01t9-1303839000-0a43d0ad0b3d302b4ee1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03dj-0222911000-ec80d66ab279caa96408 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01r7-4669400000-f144a6f4163144d3ba6a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0000902000-eabf540302c6d2302496 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0190-0000909000-0960e18ac87c6fb19ef1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-0000900000-ab6c9730050acb7a41bb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0000009000-1f8d504a98f308016336 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01t9-0000509000-7a7b4a5307be90e787c3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03di-0000900000-8848a9ed8c481276aff5 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0303591 |
|---|
| FooDB ID | FDB016391 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|