| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:12:28 UTC |
|---|
| Update Date | 2016-11-09 01:19:09 UTC |
|---|
| Accession Number | CHEM030840 |
|---|
| Identification |
|---|
| Common Name | Americanin B |
|---|
| Class | Small Molecule |
|---|
| Description | Americanin B is found in fruits. Americanin B is a constituent of the seeds of Phytolacca americana (pokeberry). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
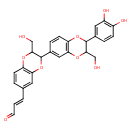
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Americanin b | MeSH |
|
|---|
| Chemical Formula | C27H24O9 |
|---|
| Average Molecular Mass | 492.474 g/mol |
|---|
| Monoisotopic Mass | 492.142 g/mol |
|---|
| CAS Registry Number | 77053-44-2 |
|---|
| IUPAC Name | (2E)-3-{3-[2-(3,4-dihydroxyphenyl)-3-(hydroxymethyl)-2,3-dihydro-1,4-benzodioxin-6-yl]-2-(hydroxymethyl)-2,3-dihydro-1,4-benzodioxin-6-yl}prop-2-enal |
|---|
| Traditional Name | (2E)-3-{3-[2-(3,4-dihydroxyphenyl)-3-(hydroxymethyl)-2,3-dihydro-1,4-benzodioxin-6-yl]-2-(hydroxymethyl)-2,3-dihydro-1,4-benzodioxin-6-yl}prop-2-enal |
|---|
| SMILES | OCC1OC2=C(OC1C1=CC3=C(OC(C(CO)O3)C3=CC(O)=C(O)C=C3)C=C1)C=C(\C=C\C=O)C=C2 |
|---|
| InChI Identifier | InChI=1S/C27H24O9/c28-9-1-2-15-3-7-20-22(10-15)36-27(24(13-29)33-20)17-5-8-21-23(12-17)34-25(14-30)26(35-21)16-4-6-18(31)19(32)11-16/h1-12,24-27,29-32H,13-14H2/b2-1+ |
|---|
| InChI Key | VDIFGESBHJLSFS-OWOJBTEDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylbenzo-1,4-dioxanes. These are benzo-1,3-dioxanes having a phenyl group attached to the 1,4-dioxane moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzodioxanes |
|---|
| Sub Class | Phenylbenzodioxanes |
|---|
| Direct Parent | Phenylbenzo-1,4-dioxanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - 2-phenylbenzo-1,4-dioxane
- Benzo-1,4-dioxane
- Catechol
- Styrene
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Phenol
- Monocyclic benzene moiety
- Para-dioxin
- Benzenoid
- Enal
- Alpha,beta-unsaturated aldehyde
- Ether
- Oxacycle
- Aldehyde
- Organooxygen compound
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Alcohol
- Organic oxygen compound
- Primary alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-03di-0210900000-beca3e99c3d206d4bdd9 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00di-6200049000-29d3e45ed245967481a4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002f-0312900000-64475446763fa1c909de | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-002b-0210900000-745e78b9e00aea8746f3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000t-0910000000-359177e9fdd57650c3ea | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0000900000-a4492fbdd249415c899b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0096-0402900000-dbd90a9592280f063f28 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kb-1920200000-21bd31af98517a0dbd56 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0001900000-98c0719f6022e9bd138a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0112900000-21cee2262248e02e822a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0019-0322900000-ad6c912b7edd52020b98 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0003900000-5de809821d9f6bbc4708 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0037-0002900000-c1264c0170936e1c941f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-009f-0625900000-e560d70ff502cb64ad9f | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0037338 |
|---|
| FooDB ID | FDB016360 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00055486 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 30839456 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 73352644 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|