| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:11:21 UTC |
|---|
| Update Date | 2016-11-09 01:19:09 UTC |
|---|
| Accession Number | CHEM030816 |
|---|
| Identification |
|---|
| Common Name | Jubanine C |
|---|
| Class | Small Molecule |
|---|
| Description | Jubanine C is found in fruits. Jubanine C is an alkaloid from the stem bark of Zizyphus jujuba (Chinese date). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
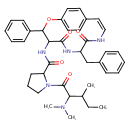
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| N-[(10Z)-7-Benzyl-5,8-dihydroxy-3-phenyl-2-oxa-6,9-diazabicyclo[10.2.2]hexadeca-1(14),5,8,10,12,15-hexaen-4-yl]-1-[2-(dimethylamino)-3-methylpentanoyl]pyrrolidine-2-carboximidate | HMDB | | Jubanine-C | HMDB | | Jubanine C | MeSH |
|
|---|
| Chemical Formula | C39H47N5O5 |
|---|
| Average Molecular Mass | 665.821 g/mol |
|---|
| Monoisotopic Mass | 665.358 g/mol |
|---|
| CAS Registry Number | 159226-00-3 |
|---|
| IUPAC Name | N-[(10Z)-7-benzyl-5,8-dioxo-3-phenyl-2-oxa-6,9-diazabicyclo[10.2.2]hexadeca-1(14),10,12,15-tetraen-4-yl]-1-[2-(dimethylamino)-3-methylpentanoyl]pyrrolidine-2-carboxamide |
|---|
| Traditional Name | N-[(10Z)-7-benzyl-5,8-dioxo-3-phenyl-2-oxa-6,9-diazabicyclo[10.2.2]hexadeca-1(14),10,12,15-tetraen-4-yl]-1-[2-(dimethylamino)-3-methylpentanoyl]pyrrolidine-2-carboxamide |
|---|
| SMILES | CCC(C)C(N(C)C)C(=O)N1CCCC1C(=O)NC1C(OC2=CC=C(C=C2)\C=C/NC(=O)C(CC2=CC=CC=C2)NC1=O)C1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C39H47N5O5/c1-5-26(2)34(43(3)4)39(48)44-24-12-17-32(44)37(46)42-33-35(29-15-10-7-11-16-29)49-30-20-18-27(19-21-30)22-23-40-36(45)31(41-38(33)47)25-28-13-8-6-9-14-28/h6-11,13-16,18-23,26,31-35H,5,12,17,24-25H2,1-4H3,(H,40,45)(H,41,47)(H,42,46)/b23-22- |
|---|
| InChI Key | JMILOTKBOBTKBB-FCQUAONHSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Oligopeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-oligopeptide
- Cyclic alpha peptide
- Isoleucine or derivatives
- Macrolactam
- Proline or derivatives
- N-acyl-alpha amino acid or derivatives
- Alpha-amino acid amide
- Alpha-amino acid or derivatives
- N-acylpyrrolidine
- Pyrrolidine carboxylic acid or derivatives
- Pyrrolidine-2-carboxamide
- Alkyl aryl ether
- Benzenoid
- Monocyclic benzene moiety
- Tertiary carboxylic acid amide
- Pyrrolidine
- Tertiary aliphatic amine
- Tertiary amine
- Secondary carboxylic acid amide
- Amino acid or derivatives
- Carboxamide group
- Lactam
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Ether
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Carbonyl group
- Hydrocarbon derivative
- Organonitrogen compound
- Amine
- Organooxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0r00-9620030000-26b1cca4888703995883 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00or-0210829000-bd3dd28c089b83b496a5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03fr-5900600000-825163d37baabd3205c7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-02ml-9200100000-bb05a98195a862c71776 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0010129000-e598938481de80784655 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03mi-1341697000-083ab9b7f0b5e04ab193 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03mj-9702400000-58081803a63ed0b9f803 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0000029000-af7290061c41c5d324a5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-9200038000-e7cc53dc1077e3118354 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9100000000-16994bff47579c687bab | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0000039000-4d31cfb331ac5f78b60f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ir0-3100698000-bddda65f833471d9177e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002f-9605300000-7412c092b5d1b6d7e11e | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0037300 |
|---|
| FooDB ID | FDB016319 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00027401 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35014397 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131752168 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|