| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:11:17 UTC |
|---|
| Update Date | 2016-11-09 01:19:08 UTC |
|---|
| Accession Number | CHEM030813 |
|---|
| Identification |
|---|
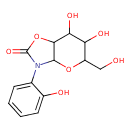
| Common Name | Hexahydro-6,7-dihydroxy-5-(hydroxymethyl)-3-(2-hydroxyphenyl)-2H-pyrano[2,3-d]oxazol-2-one |
|---|
| Class | Small Molecule |
|---|
| Description | Hexahydro-6,7-dihydroxy-5-(hydroxymethyl)-3-(2-hydroxyphenyl)-2H-pyrano[2,3-d]oxazol-2-one is found in cereals and cereal products. Hexahydro-6,7-dihydroxy-5-(hydroxymethyl)-3-(2-hydroxyphenyl)-2H-pyrano[2,3-d]oxazol-2-one is isolated from the roots of oats (Avena sativa) and corn (Zea mays). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-(2-hydroxyphenylamino)-1-Deoxyglucoside 1,2-carbamate | HMDB |
|
|---|
| Chemical Formula | C13H15NO7 |
|---|
| Average Molecular Mass | 297.261 g/mol |
|---|
| Monoisotopic Mass | 297.085 g/mol |
|---|
| CAS Registry Number | 396714-67-3 |
|---|
| IUPAC Name | 6,7-dihydroxy-5-(hydroxymethyl)-3-(2-hydroxyphenyl)-hexahydro-2H-pyrano[2,3-d][1,3]oxazol-2-one |
|---|
| Traditional Name | 6,7-dihydroxy-5-(hydroxymethyl)-3-(2-hydroxyphenyl)-tetrahydro-3aH-pyrano[2,3-d][1,3]oxazol-2-one |
|---|
| SMILES | OCC1OC2C(OC(=O)N2C2=CC=CC=C2O)C(O)C1O |
|---|
| InChI Identifier | InChI=1S/C13H15NO7/c15-5-8-9(17)10(18)11-12(20-8)14(13(19)21-11)6-3-1-2-4-7(6)16/h1-4,8-12,15-18H,5H2 |
|---|
| InChI Key | XBYMZYWXQBJAJG-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1-hydroxy-4-unsubstituted benzenoids. These are phenols that are unsubstituted at the 4-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Phenols |
|---|
| Sub Class | 1-hydroxy-4-unsubstituted benzenoids |
|---|
| Direct Parent | 1-hydroxy-4-unsubstituted benzenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Monocyclic benzene moiety
- Monosaccharide
- Oxane
- Oxazolidinone
- Oxazolidine
- Carbamic acid ester
- Carbonic acid derivative
- Secondary alcohol
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Alcohol
- Primary alcohol
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Carbonyl group
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05ui-2590000000-3d89132d38927d69c2b7 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-0229-2029260000-e3f5284871cf4c278cf3 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0090000000-69737ccccb9ced503c15 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000w-9260000000-7ffd48648492b5759f8a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fdo-9320000000-c8d377ffdb53418079aa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0390000000-62f08a7bac25c4b2681f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-056r-1950000000-2c07e7a7937070ce1cb5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-5900000000-6e3dd509da646083ce41 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-f290dd3d66fa5cec873b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a6u-1920000000-8ca4c0ec55fdeb99890e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-2910000000-364aeb71bddd317f597e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0090000000-f794a34099a22df614a6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0490000000-e27585fe66531d21ee47 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052b-2900000000-7462136131b859218308 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0037297 |
|---|
| FooDB ID | FDB016316 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 20056919 |
|---|
| ChEBI ID | 174830 |
|---|
| PubChem Compound ID | 22297162 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|