| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:11:13 UTC |
|---|
| Update Date | 2016-11-09 01:19:08 UTC |
|---|
| Accession Number | CHEM030812 |
|---|
| Identification |
|---|
| Common Name | 3-Acetyl-2,7-naphthyridine |
|---|
| Class | Small Molecule |
|---|
| Description | 3-Acetyl-2,7-naphthyridine is found in fats and oils. 3-Acetyl-2,7-naphthyridine is an alkaloid from valerian (Valeriana officinalis). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
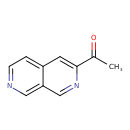
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2,7-Naphthyridine-3-methylketone | MeSH | | 1-(2,7-Naphthyridin-3-yl)-ethanone | HMDB | | 1-(2,7-Naphthyridin-3-yl)ethanone, 9ci | HMDB | | Naphthyridylmethylketone | HMDB | | 3-Acetyl-2,7-naphthyridine | MeSH |
|
|---|
| Chemical Formula | C10H8N2O |
|---|
| Average Molecular Mass | 172.183 g/mol |
|---|
| Monoisotopic Mass | 172.064 g/mol |
|---|
| CAS Registry Number | 73607-00-8 |
|---|
| IUPAC Name | 1-(2,7-naphthyridin-3-yl)ethan-1-one |
|---|
| Traditional Name | 1-(2,7-naphthyridin-3-yl)ethanone |
|---|
| SMILES | CC(=O)C1=CC2=CC=NC=C2C=N1 |
|---|
| InChI Identifier | InChI=1S/C10H8N2O/c1-7(13)10-4-8-2-3-11-5-9(8)6-12-10/h2-6H,1H3 |
|---|
| InChI Key | VBBXXOOMKKQNNS-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as naphthyridines. Naphthyridines are compounds containing a naphthyridine moiety, a naphthalene in which a carbon atom has been replaced by a nitrogen in each of the two rings. The naphthyridine skeleton can also be described as an assembly two fused pyridine rings, which do not share their nitrogen atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Diazanaphthalenes |
|---|
| Sub Class | Naphthyridines |
|---|
| Direct Parent | Naphthyridines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Naphthyridine
- Aryl alkyl ketone
- Aryl ketone
- Pyridine
- Heteroaromatic compound
- Ketone
- Azacycle
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-059x-3900000000-a5c243d3eb72418f8520 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0900000000-41cedb7d00e7b7eac843 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05fr-0900000000-0ac3e990c7c5e207d6d0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-0900000000-45039c2c9af2fac467aa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0900000000-d8c9c01ec14da1e39852 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0900000000-114ec2b9ccf8dde40d39 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-0900000000-289b6c39e18fe799583e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00fr-0900000000-9336d9e8875928740d21 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0900000000-4d63a8d640822047a23f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fb9-1900000000-99f2d8e812f3055a04fc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0900000000-95370f49b668a829b381 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0900000000-f7eea75e709aa772f169 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ufr-1900000000-89d3977a916ba0276111 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0037296 |
|---|
| FooDB ID | FDB016315 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00054660 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 137230 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 155795 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|