| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:11:04 UTC |
|---|
| Update Date | 2016-11-09 01:19:08 UTC |
|---|
| Accession Number | CHEM030810 |
|---|
| Identification |
|---|
| Common Name | 5(1->10)-Abeo-1,12-patchoulanediol |
|---|
| Class | Small Molecule |
|---|
| Description | Constituent of Pogostemon cablin (patchouli). 5(1->10)-Abeo-1,12-patchoulanediol is found in herbs and spices. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
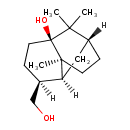
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C15H26O2 |
|---|
| Average Molecular Mass | 238.366 g/mol |
|---|
| Monoisotopic Mass | 238.193 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (1R,3S,6S,7S,8S)-6-(hydroxymethyl)-2,2,8-trimethyltricyclo[5.3.1.0³,⁸]undecan-3-ol |
|---|
| Traditional Name | (1R,3S,6S,7S,8S)-6-(hydroxymethyl)-2,2,8-trimethyltricyclo[5.3.1.0³,⁸]undecan-3-ol |
|---|
| SMILES | [H][C@@]12C[C@H]3CC[C@]1(C)[C@](O)(CC[C@@H]2CO)C3(C)C |
|---|
| InChI Identifier | InChI=1S/C15H26O2/c1-13(2)11-5-6-14(3)12(8-11)10(9-16)4-7-15(13,14)17/h10-12,16-17H,4-9H2,1-3H3/t10-,11-,12+,14+,15+/m1/s1 |
|---|
| InChI Key | RTSORXBIZDRAMP-MUGBGTHKSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tertiary alcohols. Tertiary alcohols are compounds in which a hydroxy group, -OH, is attached to a saturated carbon atom R3COH (R not H ). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Alcohols and polyols |
|---|
| Direct Parent | Tertiary alcohols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tertiary alcohol
- Cyclic alcohol
- Hydrocarbon derivative
- Primary alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00vi-9860000000-3806c6230c5f0f6ba881 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-01dr-9135000000-320bcf9d311630b46ecd | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00dr-0090000000-f4fbd5ea067356e9aa8f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fk9-0190000000-ee52ff9b4d4835cb7b52 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udl-3960000000-2854d2b21a4ba9eb2033 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-771218ebab04c1193da7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00kr-0190000000-c34dbe18d989138088e2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4u-1970000000-8d39aa651dab86092af0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0090000000-f9d1e2ca69aa56a5a13c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0gbi-0590000000-b2326c3ced0b1d460af6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-066u-9820000000-5a0359d45249e6a6e066 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-e665f494a7390ebf108d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0090000000-e665f494a7390ebf108d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-0090000000-e7c7c1fef4e272f35016 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0037284 |
|---|
| FooDB ID | FDB016303 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|