| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:10:23 UTC |
|---|
| Update Date | 2016-11-09 01:19:08 UTC |
|---|
| Accession Number | CHEM030796 |
|---|
| Identification |
|---|
| Common Name | Haem |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
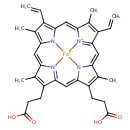
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| [3,7,12,17-Tetramethyl-8,13-divinylporphyrin-2,18-dipropanoato(2-)]iron(II) | ChEBI | | [Fe(ppix)] | ChEBI | | Fe(ppix) | ChEBI | | Ferroprotoheme | ChEBI | | Ferroprotoporphyrin IX | ChEBI | | Ferrous protoheme | ChEBI | | Ferrous protoheme IX | ChEBI | | Haem | ChEBI | | Iron(II) protoporphyrin IX | ChEBI | | Protoferroheme | ChEBI | | Protoheme | ChEBI | | Heme b | Kegg | | Protoheme IX | Kegg | | Heme | ChEBI |
|
|---|
| Chemical Formula | C34H32FeN4O4 |
|---|
| Average Molecular Mass | 616.487 g/mol |
|---|
| Monoisotopic Mass | 616.177 g/mol |
|---|
| CAS Registry Number | 14875-96-8 |
|---|
| IUPAC Name | 4,20-bis(2-carboxyethyl)-10,15-diethenyl-5,9,14,19-tetramethyl-2lambda5,22,23lambda5,25-tetraaza-1-ferraoctacyclo[11.9.1.1^{1,8}.1^{3,21}.0^{2,6}.0^{16,23}.0^{18,22}.0^{11,25}]pentacosa-2,4,6,8,10,12,14,16(23),17,19,21(24)-undecaene-2,23-bis(ylium)-1,1-diuide |
|---|
| Traditional Name | 4,20-bis(2-carboxyethyl)-10,15-diethenyl-5,9,14,19-tetramethyl-2lambda5,22,23lambda5,25-tetraaza-1-ferraoctacyclo[11.9.1.1^{1,8}.1^{3,21}.0^{2,6}.0^{16,23}.0^{18,22}.0^{11,25}]pentacosa-2,4,6,8,10,12,14,16(23),17,19,21(24)-undecaene-2,23-bis(ylium)-1,1-diuide |
|---|
| SMILES | CC1=C(CCC(O)=O)C2=CC3=[N]4C(=CC5=C(C)C(C=C)=C6C=C7C(C)=C(C=C)C8=[N]7[Fe]4(N2C1=C8)N56)C(C)=C3CCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C34H34N4O4.Fe/c1-7-21-17(3)25-13-26-19(5)23(9-11-33(39)40)31(37-26)16-32-24(10-12-34(41)42)20(6)28(38-32)15-30-22(8-2)18(4)27(36-30)14-29(21)35-25;/h7-8,13-16H,1-2,9-12H2,3-6H3,(H4,35,36,37,38,39,40,41,42);/q;+2/p-2/b25-13-,26-13-,27-14-,28-15-,29-14-,30-15-,31-16-,32-16-; |
|---|
| InChI Key | KABFMIBPWCXCRK-RGGAHWMASA-L |
|---|
| Chemical Taxonomy |
|---|
| Classification | Not classified |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("Heme,1TMS,#1" TMS) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-066r-0000095000-501c4a776d38d61ab89a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0gi4-0000092000-64c21d0a0f8daa069fb9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03gs-4000590000-15136ce22e51bdc57377 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0000049000-69ee302652cebc40f13b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00r2-1000091000-d178bb83079a203a48dc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-1000090000-f6e437751fbc08f12d1f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014j-0000059000-0ab427d87ba10992d498 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0000098000-650f7d2f023028ff2319 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-06fr-0000090000-2dff1da74eb12a246237 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014j-0000079000-990502ea40621d9cad46 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05ns-0000091000-3547d5c809ccf8987200 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0cdr-0000092000-e55d1ab39076367d5b9e | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0003178 |
|---|
| FooDB ID | FDB031136 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Heme |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 17627 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | C00032 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | ECMDB03178 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Ono F, Sharma BK, Smith CC, Burnett JW, Aurelian L: CD34+ cells in the peripheral blood transport herpes simplex virus DNA fragments to the skin of patients with erythema multiforme (HAEM). J Invest Dermatol. 2005 Jun;124(6):1215-24. | | 2. Allhorn M, Lundqvist K, Schmidtchen A, Akerstrom B: Heme-scavenging role of alpha1-microglobulin in chronic ulcers. J Invest Dermatol. 2003 Sep;121(3):640-6. | | 3. Walczyk T, von Blanckenburg F: Natural iron isotope variations in human blood. Science. 2002 Mar 15;295(5562):2065-6. | | 4. Kuhnel A, Gross U, Doss MO: Hereditary coproporphyria in Germany: clinical-biochemical studies in 53 patients. Clin Biochem. 2000 Aug;33(6):465-73. | | 5. Zhang JP, Normark S: Induction of gene expression in Escherichia coli after pilus-mediated adherence. Science. 1996 Aug 30;273(5279):1234-6. | | 6. Taylor TD, Litt M, Kramer P, Pandolfo M, Angelini L, Nardocci N, Davis S, Pineda M, Hattori H, Flett PJ, Cilio MR, Bertini E, Hayflick SJ: Homozygosity mapping of Hallervorden-Spatz syndrome to chromosome 20p12.3-p13. Nat Genet. 1996 Dec;14(4):479-81. | | 7. Park KK, Park JH, Jung YJ, Chung WY: Inhibitory effects of chlorophyllin, hemin and tetrakis(4-benzoic acid)porphyrin on oxidative DNA damage and mouse skin inflammation induced by 12-O-tetradecanoylphorbol-13-acetate as a possible anti-tumor promoting mechanism. Mutat Res. 2003 Dec 9;542(1-2):89-97. | | 8. Elshenawy S, Pinney SE, Stuart T, Doulias PT, Zura G, Parry S, Elovitz MA, Bennett MJ, Bansal A, Strauss JF 3rd, Ischiropoulos H, Simmons RA: The Metabolomic Signature of the Placenta in Spontaneous Preterm Birth. Int J Mol Sci. 2020 Feb 4;21(3). pii: ijms21031043. doi: 10.3390/ijms21031043. | | 9. https://www.ncbi.nlm.nih.gov/pubmed/?term=20546754 |
|
|---|