| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:08:42 UTC |
|---|
| Update Date | 2016-11-09 01:19:08 UTC |
|---|
| Accession Number | CHEM030762 |
|---|
| Identification |
|---|
| Common Name | (2R)-2-Hydroxy-2-phenylethyl glucosinolate |
|---|
| Class | Small Molecule |
|---|
| Description | (2S)-2-Hydroxy-2-phenylethyl glucosinolate is found in brassicas. (2S)-2-Hydroxy-2-phenylethyl glucosinolate is a constituent of Barbarea vulgaris (winter cress). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
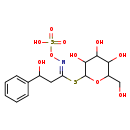
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (2R)-2-Hydroxy-2-phenylethyl glucosinolic acid | Generator | | {[(e)-(3-hydroxy-3-phenyl-1-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]sulfanyl}propylidene)amino]oxy}sulfonate | HMDB | | {[(e)-(3-hydroxy-3-phenyl-1-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]sulphanyl}propylidene)amino]oxy}sulphonate | HMDB | | {[(e)-(3-hydroxy-3-phenyl-1-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]sulphanyl}propylidene)amino]oxy}sulphonic acid | HMDB |
|
|---|
| Chemical Formula | C15H21NO10S2 |
|---|
| Average Molecular Mass | 439.458 g/mol |
|---|
| Monoisotopic Mass | 439.061 g/mol |
|---|
| CAS Registry Number | 37913-31-8 |
|---|
| IUPAC Name | {[(E)-(3-hydroxy-3-phenyl-1-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]sulfanyl}propylidene)amino]oxy}sulfonic acid |
|---|
| Traditional Name | [(E)-(3-hydroxy-3-phenyl-1-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]sulfanyl}propylidene)amino]oxysulfonic acid |
|---|
| SMILES | OCC1OC(S\C(CC(O)C2=CC=CC=C2)=N\OS(O)(=O)=O)C(O)C(O)C1O |
|---|
| InChI Identifier | InChI=1S/C15H21NO10S2/c17-7-10-12(19)13(20)14(21)15(25-10)27-11(16-26-28(22,23)24)6-9(18)8-4-2-1-3-5-8/h1-5,9-10,12-15,17-21H,6-7H2,(H,22,23,24)/b16-11+ |
|---|
| InChI Key | GAPDDBFHNYHZIS-LFIBNONCSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as alkylglucosinolates. These are organic compounds containing a glucosinolate moiety that carries an alkyl chain. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Alkylglucosinolates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alkylglucosinolate
- Glycosyl compound
- S-glycosyl compound
- Monocyclic benzene moiety
- Oxane
- Benzenoid
- Monothioacetal
- Organic sulfuric acid or derivatives
- Secondary alcohol
- Organoheterocyclic compound
- Polyol
- Oxacycle
- Sulfenyl compound
- Aromatic alcohol
- Alcohol
- Primary alcohol
- Organosulfur compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic nitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0ab9-4901300000-fc14fc30288077fc2ee7 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-004l-2920024000-706814132504ea75714c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00dm-0540900000-8a6640d11fb776a74c9a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a6r-0539000000-eed55bb83c345829745a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0abc-9330000000-36e7995c9133ff312542 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0570-3291100000-a0b1433ec5d7457309b0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0706-6890000000-8e12ccd7b950554c4e2b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01vo-8950000000-ca6259d7a874ad4baae1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0000900000-161900bbee438f9884d0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-006x-0214900000-44bef02aa9161039b1c7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0adi-2936000000-b399bb170525e3cebe7d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0300900000-fa5f2c122584a6e90f40 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-2925200000-5a59f6eff19348bf5689 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-1000-0910000000-b7971bf0a2c8b4b15b0a | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0037205 |
|---|
| FooDB ID | FDB016210 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35014388 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 12897776 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|