| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:08:15 UTC |
|---|
| Update Date | 2016-11-09 01:19:08 UTC |
|---|
| Accession Number | CHEM030754 |
|---|
| Identification |
|---|
| Common Name | Polysorbate 60 |
|---|
| Class | Small Molecule |
|---|
| Description | Polysorbate 60 is an emulsifier, opacifier, protective coating, dough conditioner, dispersant, wetting agent, stabiliser, defoamer, poultry scald agent, flavour.Polysorbates are a class of emulsifiers used in some pharmaceuticals and food preparation. They are often used in cosmetics to solubilize essential oils into water-based products. Polysorbates are oily liquids derived from PEG-ylated sorbitan (a derivative of sorbitol) esterified with fatty acids. (Wikipedia). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
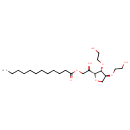
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Polysorbic acid 60 | Generator | | e435 | HMDB | | PEG-60 sorbitan stearate | HMDB | | POE(20) sorbitan monostearate | HMDB | | Polyoxyethylene (60) sorbitan monostearate | HMDB | | Polyoxyethylene sorbitan monostearate | HMDB | | Sorbomacrogol stearate 300 | HMDB | | Tween 60 | HMDB | | (2R)-2-[(2R,3R,4S)-3,4-Bis(2-hydroxyethoxy)oxolan-2-yl]-2-hydroxyethyl dodecanoic acid | Generator |
|
|---|
| Chemical Formula | C22H42O8 |
|---|
| Average Molecular Mass | 434.564 g/mol |
|---|
| Monoisotopic Mass | 434.288 g/mol |
|---|
| CAS Registry Number | 9005-67-8 |
|---|
| IUPAC Name | (2R)-2-[(2R,3R,4S)-3,4-bis(2-hydroxyethoxy)oxolan-2-yl]-2-hydroxyethyl dodecanoate |
|---|
| Traditional Name | (2R)-2-[(2R,3R,4S)-3,4-bis(2-hydroxyethoxy)oxolan-2-yl]-2-hydroxyethyl dodecanoate |
|---|
| SMILES | CCCCCCCCCCCC(=O)OC[C@@H](O)[C@H]1OC[C@H](OCCO)[C@H]1OCCO |
|---|
| InChI Identifier | InChI=1S/C22H42O8/c1-2-3-4-5-6-7-8-9-10-11-20(26)29-16-18(25)21-22(28-15-13-24)19(17-30-21)27-14-12-23/h18-19,21-25H,2-17H2,1H3/t18-,19+,21-,22-/m1/s1 |
|---|
| InChI Key | CRBBOOXGHMTWOC-NPDDRXJXSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as fatty acid esters. These are carboxylic ester derivatives of a fatty acid. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acid esters |
|---|
| Direct Parent | Fatty acid esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Fatty acid ester
- Tetrahydrofuran
- Carboxylic acid ester
- Secondary alcohol
- Carboxylic acid derivative
- Dialkyl ether
- Ether
- Monocarboxylic acid or derivatives
- Oxacycle
- Organoheterocyclic compound
- Organic oxide
- Carbonyl group
- Alcohol
- Organooxygen compound
- Primary alcohol
- Hydrocarbon derivative
- Organic oxygen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0f76-2911100000-423640d9d28653c447dc | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-052r-3905224000-21d4f3f0ebc3da5b2f5d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-1773900000-7658305b424bfab18341 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00m3-2941100000-fca3199396b69eec48d1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0005-4915000000-c8669a6456ea00a054fa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001j-1920200000-08018bf25580e7092c3e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000t-1910000000-ad8dc934068b34929899 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03dm-9700000000-d33797d462b3ab42e574 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-3101900000-058192223c70786bcc23 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052f-9631300000-cdb5223fea10e2df2afe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-9000000000-ec1e93df781266415ef4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-003r-0109400000-700e415e1f5f5825fc23 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001l-6932600000-0cc0367d54928754a82f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0005-3910000000-4c636ca59829f3903233 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0037183 |
|---|
| FooDB ID | FDB016182 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Polysorbate |
|---|
| Chemspider ID | 17215564 |
|---|
| ChEBI ID | 53425 |
|---|
| PubChem Compound ID | 22833389 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|