| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:05:25 UTC |
|---|
| Update Date | 2016-11-09 01:19:07 UTC |
|---|
| Accession Number | CHEM030697 |
|---|
| Identification |
|---|
| Common Name | (E)-Resveratrol 3-(2''-sulfoglucoside) |
|---|
| Class | Small Molecule |
|---|
| Description | (Z)-Resveratrol 3-(2''-sulfoglucoside) is found in green vegetables. (Z)-Resveratrol 3-(2''-sulfoglucoside) is a constituent of Polygonum cuspidatum (Japanese knotweed). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
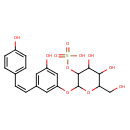
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (Z)-Resveratrol 3-(2''-sulphoglucoside) | Generator | | (4,5-Dihydroxy-2-{3-hydroxy-5-[(Z)-2-(4-hydroxyphenyl)ethenyl]phenoxy}-6-(hydroxymethyl)oxan-3-yl)oxidanesulfonate | Generator | | (4,5-Dihydroxy-2-{3-hydroxy-5-[(Z)-2-(4-hydroxyphenyl)ethenyl]phenoxy}-6-(hydroxymethyl)oxan-3-yl)oxidanesulphonate | Generator | | (4,5-Dihydroxy-2-{3-hydroxy-5-[(Z)-2-(4-hydroxyphenyl)ethenyl]phenoxy}-6-(hydroxymethyl)oxan-3-yl)oxidanesulphonic acid | Generator |
|
|---|
| Chemical Formula | C20H22O11S |
|---|
| Average Molecular Mass | 470.447 g/mol |
|---|
| Monoisotopic Mass | 470.088 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (4,5-dihydroxy-2-{3-hydroxy-5-[(Z)-2-(4-hydroxyphenyl)ethenyl]phenoxy}-6-(hydroxymethyl)oxan-3-yl)oxidanesulfonic acid |
|---|
| Traditional Name | (4,5-dihydroxy-2-{3-hydroxy-5-[(Z)-2-(4-hydroxyphenyl)ethenyl]phenoxy}-6-(hydroxymethyl)oxan-3-yl)oxidanesulfonic acid |
|---|
| SMILES | OCC1OC(OC2=CC(\C=C/C3=CC=C(O)C=C3)=CC(O)=C2)C(OS(O)(=O)=O)C(O)C1O |
|---|
| InChI Identifier | InChI=1S/C20H22O11S/c21-10-16-17(24)18(25)19(31-32(26,27)28)20(30-16)29-15-8-12(7-14(23)9-15)2-1-11-3-5-13(22)6-4-11/h1-9,16-25H,10H2,(H,26,27,28)/b2-1- |
|---|
| InChI Key | NSGOKDPIHZLAAL-UPHRSURJSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as stilbene glycosides. Stilbene glycosides are compounds structurally characterized by the presence of a carbohydrate moiety glycosidically linked to the stilbene skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Stilbenes |
|---|
| Sub Class | Stilbene glycosides |
|---|
| Direct Parent | Stilbene glycosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Stilbene glycoside
- Phenolic glycoside
- Hexose monosaccharide
- Glycosyl compound
- O-glycosyl compound
- Phenoxy compound
- Phenol ether
- Styrene
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- 1-hydroxy-4-unsubstituted benzenoid
- Monocyclic benzene moiety
- Monosaccharide
- Oxane
- Benzenoid
- Sulfuric acid ester
- Sulfuric acid monoester
- Sulfate-ester
- Alkyl sulfate
- Organic sulfuric acid or derivatives
- Secondary alcohol
- Oxacycle
- Organoheterocyclic compound
- Acetal
- Organooxygen compound
- Primary alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Organic oxide
- Alcohol
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0fer-7951600000-e347c62a649467b41e2a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-00di-3813009000-844345d40aea4c5c21f8 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00b9-0093400000-c5464c63aaa409a3e10b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0191000000-36da20ea8ca7140cdf62 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01t9-1980000000-950f92ee74636c891871 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-016r-1081900000-8f0952587750c538ed91 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-1191100000-c1df38ea33e6c0e16f5a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-2490000000-ce00972a81031a14bc56 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00fr-0070900000-6870ea75d49be47a942a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00b9-2792500000-8d282c210c88bd7a237c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-002r-5950100000-4dd1641a811f586030cd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0000900000-ef902abb3c9334758f2d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0291000000-f793720e79937fe16b17 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-003s-9460200000-7b667743dd9bce580d32 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0037075 |
|---|
| FooDB ID | FDB016063 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131752140 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|