| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:05:14 UTC |
|---|
| Update Date | 2016-11-09 01:19:07 UTC |
|---|
| Accession Number | CHEM030692 |
|---|
| Identification |
|---|
| Common Name | Sporol |
|---|
| Class | Small Molecule |
|---|
| Description | Sporol is a mycotoxin from Fusarium sporotrichioides MC-72083. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
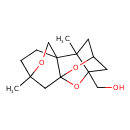
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Helminthosporol | HMDB | | [3S-(3alpha,4Abeta,5aalpha,7b,8aalpha,8balpha)]-hexahydro-3,8a-dimethyl-4a,7-epoxy-3,8b-ethano-1H,5ah-cyclopenta[4,5]furo[3,2-c]pyran-5a-methanol | HMDB |
|
|---|
| Chemical Formula | C15H22O4 |
|---|
| Average Molecular Mass | 266.333 g/mol |
|---|
| Monoisotopic Mass | 266.152 g/mol |
|---|
| CAS Registry Number | 101401-88-1 |
|---|
| IUPAC Name | {2,10-dimethyl-7,11,15-trioxapentacyclo[8.2.2.1⁴,⁸.0¹,⁸.0²,⁶]pentadecan-6-yl}methanol |
|---|
| Traditional Name | {2,10-dimethyl-7,11,15-trioxapentacyclo[8.2.2.1⁴,⁸.0¹,⁸.0²,⁶]pentadecan-6-yl}methanol |
|---|
| SMILES | CC12CC3CC1(CO)OC1(CC4(C)CCC21CO4)O3 |
|---|
| InChI Identifier | InChI=1S/C15H22O4/c1-11-3-4-13(9-17-11)12(2)5-10-6-14(12,8-16)19-15(13,7-11)18-10/h10,16H,3-9H2,1-2H3 |
|---|
| InChI Key | RGGZJNLZRGIMHQ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1,3-dioxepanes. These are dioxepanes with the two ring oxygen atoms at position 1 and 3, respectively. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Dioxepanes |
|---|
| Sub Class | 1,3-dioxepanes |
|---|
| Direct Parent | 1,3-dioxepanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Ketal
- 1,3-dioxepane
- Oxepane
- Meta-dioxane
- Oxane
- Tetrahydrofuran
- Acetal
- Dialkyl ether
- Ether
- Oxacycle
- Hydrocarbon derivative
- Organic oxygen compound
- Organooxygen compound
- Alcohol
- Primary alcohol
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05q0-3090000000-3f5af3e6c192029dada1 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0fki-9342000000-11f71c0b5da32d7ba5ab | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0090000000-9418dcc929f9208ed02d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014j-0090000000-f5ed509b53292dcc552a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001j-0090000000-e77c174632a9987f0dd5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0090000000-4559bbf4c8bcd2d88cd0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0090000000-2e4bb45fc3b7db50ee30 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014j-0090000000-75cf302903f84ceb24fe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0090000000-f9a94f566277e5f223eb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0090000000-085aa2a7c66a1b8c4b12 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-0090000000-9f5a392f712dfb76dfb6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0090000000-c6cc318d73093d112373 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0090000000-c6cc318d73093d112373 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-0090000000-9365f495a5a104999821 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0037070 |
|---|
| FooDB ID | FDB016054 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00003141 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 52085311 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 71332574 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|