| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:04:14 UTC |
|---|
| Update Date | 2016-11-09 01:19:07 UTC |
|---|
| Accession Number | CHEM030668 |
|---|
| Identification |
|---|
| Common Name | Melleolide D |
|---|
| Class | Small Molecule |
|---|
| Description | Melleolide D is found in mushrooms. Melleolide D is from Armillaria mellea (honey mushroom). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
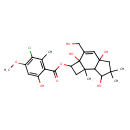
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2a,4a,7-Trihydroxy-3-(hydroxymethyl)-6,6,7b-trimethyl-1H,2H,2ah,4ah,5H,6H,7H,7ah,7BH-cyclobuta[e]inden-2-yl 3-chloro-6-hydroxy-4-methoxy-2-methylbenzoic acid | HMDB |
|
|---|
| Chemical Formula | C24H31ClO8 |
|---|
| Average Molecular Mass | 482.951 g/mol |
|---|
| Monoisotopic Mass | 482.171 g/mol |
|---|
| CAS Registry Number | 101922-80-9 |
|---|
| IUPAC Name | 2a,4a,7-trihydroxy-3-(hydroxymethyl)-6,6,7b-trimethyl-1H,2H,2aH,4aH,5H,6H,7H,7aH,7bH-cyclobuta[e]inden-2-yl 3-chloro-6-hydroxy-4-methoxy-2-methylbenzoate |
|---|
| Traditional Name | 2a,4a,7-trihydroxy-3-(hydroxymethyl)-6,6,7b-trimethyl-1H,2H,5H,7H,7aH-cyclobuta[e]inden-2-yl 3-chloro-6-hydroxy-4-methoxy-2-methylbenzoate |
|---|
| SMILES | COC1=C(Cl)C(C)=C(C(=O)OC2CC3(C)C4C(O)C(C)(C)CC4(O)C=C(CO)C23O)C(O)=C1 |
|---|
| InChI Identifier | InChI=1S/C24H31ClO8/c1-11-16(13(27)6-14(32-5)17(11)25)20(29)33-15-8-22(4)18-19(28)21(2,3)10-23(18,30)7-12(9-26)24(15,22)31/h6-7,15,18-19,26-28,30-31H,8-10H2,1-5H3 |
|---|
| InChI Key | PSCSRVBGZZBKIW-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as melleolides and analogues. Melleolides and analogues are compounds with a structure characterized by the presence of a 2-hydroxy-4-methoxy-6-methylbenzoic acid derivative linked to a 3,6,6,7b-tetramethyl-cyclobuta[e]indene moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesquiterpenoids |
|---|
| Direct Parent | Melleolides and analogues |
|---|
| Alternative Parents | |
|---|
| Substituents | - Melleolide-skeleton
- O-hydroxybenzoic acid ester
- P-methoxybenzoic acid or derivatives
- Methoxyphenol
- Benzoate ester
- Salicylic acid or derivatives
- 3-halobenzoic acid or derivatives
- Halobenzoic acid or derivatives
- Benzoic acid or derivatives
- 4-halophenol
- Phenoxy compound
- M-cresol
- Benzoyl
- 4-chlorophenol
- Anisole
- Phenol ether
- Methoxybenzene
- Phenol
- Chlorobenzene
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Halobenzene
- Toluene
- Aryl chloride
- Monocyclic benzene moiety
- Aryl halide
- Benzenoid
- Cyclic alcohol
- Tertiary alcohol
- Vinylogous acid
- Secondary alcohol
- Carboxylic acid ester
- Cyclobutanol
- Carboxylic acid derivative
- Polyol
- Monocarboxylic acid or derivatives
- Ether
- Organohalogen compound
- Organic oxygen compound
- Organochloride
- Organooxygen compound
- Primary alcohol
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0zfr-9770200000-cd835bee892f8eda2fb9 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-001i-0009001000-c8f9d1ab528d6db8a5ee | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01q9-0120900000-67b73425bc983744ffa9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-02h9-0340900000-2cca99ac94de2bbb9fbf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-1940000000-767285673ee02b563a8e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0110900000-afb66b80873564ba3c33 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0089-0741900000-c52115722c8731e36c35 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-053r-9710200000-5c3ed2e46d4e7ffaf436 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kb-0120900000-439a2ebdef09a1b62216 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0440900000-86f39c5b1eab7a3451bd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kb-1950100000-aa58e810811312b20323 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00l2-0240900000-10004e665fb7580c6c5c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0950500000-8b1e5523b6b562fe163d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00or-0970200000-6b4f1a4c26c67a1eee62 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0037042 |
|---|
| FooDB ID | FDB016024 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00021462 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35014357 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 14166115 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|