| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:03:36 UTC |
|---|
| Update Date | 2016-11-09 01:19:07 UTC |
|---|
| Accession Number | CHEM030654 |
|---|
| Identification |
|---|
| Common Name | Deoxyloganic acid |
|---|
| Class | Small Molecule |
|---|
| Description | Deoxyloganic acid is found in herbs and spices. Deoxyloganic acid is a constituent of Nepeta cataria (catnip). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
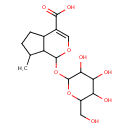
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Deoxyloganate | Generator | | 1,5,9-Epideoxyloganic acid | HMDB | | 5-Epideoxyloganic acid | HMDB | | 8-Epideoxyloganic acid | HMDB | | Deoxyloganic acid tetraacetate | HMDB | | 7-Methyl-1-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-1H,4ah,5H,6H,7H,7ah-cyclopenta[c]pyran-4-carboxylate | Generator | | 7-Deoxyloganic acid | MeSH | | Deoxyloganic acid | MeSH |
|
|---|
| Chemical Formula | C16H24O9 |
|---|
| Average Molecular Mass | 360.356 g/mol |
|---|
| Monoisotopic Mass | 360.142 g/mol |
|---|
| CAS Registry Number | 92842-56-3 |
|---|
| IUPAC Name | 7-methyl-1-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-1H,4aH,5H,6H,7H,7aH-cyclopenta[c]pyran-4-carboxylic acid |
|---|
| Traditional Name | 7-methyl-1-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-1H,4aH,5H,6H,7H,7aH-cyclopenta[c]pyran-4-carboxylic acid |
|---|
| SMILES | CC1CCC2C1C(OC1OC(CO)C(O)C(O)C1O)OC=C2C(O)=O |
|---|
| InChI Identifier | InChI=1S/C16H24O9/c1-6-2-3-7-8(14(21)22)5-23-15(10(6)7)25-16-13(20)12(19)11(18)9(4-17)24-16/h5-7,9-13,15-20H,2-4H2,1H3,(H,21,22) |
|---|
| InChI Key | DSXFHNSGLYXPNG-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as iridoid o-glycosides. These are iridoid monoterpenes containing a glycosyl (usually a pyranosyl) moiety linked to the iridoid skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Terpene glycosides |
|---|
| Direct Parent | Iridoid O-glycosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Iridoid o-glycoside
- Hexose monosaccharide
- Glycosyl compound
- Iridoid-skeleton
- O-glycosyl compound
- Bicyclic monoterpenoid
- Monoterpenoid
- Monosaccharide
- Oxane
- Vinylogous ester
- Secondary alcohol
- Acetal
- Organoheterocyclic compound
- Monocarboxylic acid or derivatives
- Oxacycle
- Carboxylic acid
- Carboxylic acid derivative
- Polyol
- Primary alcohol
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Organic oxygen compound
- Alcohol
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001l-9243000000-43372982d3a9b122d7ac | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-001i-4262149000-7f06b76b28466a7a47ed | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 6V, Negative | splash10-052b-0902000000-11d93cbeb8af54b8be2a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03ea-0903000000-903f3233a9d004c8a670 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0gwb-0900000000-16a2a61f6e5048510028 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f89-8900000000-d5581b1774594a18f3ac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0k92-1916000000-085a5cc78cd6cef3002f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0gvk-2901000000-1d6ff12cb7f9a07d4ede | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-02pm-5900000000-eacdc982c84260461c14 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03e9-0905000000-bb25e21a7bce7031ca95 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01q9-1901000000-ade8544912ace44459cb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000t-9600000000-bde85ac4775cebdf5d57 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0109000000-daebe79714eeb047d4f1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0pb9-3916000000-30a970c565499ecb61bb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a6u-6910000000-812f9f177e9f0c38daa8 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0037028 |
|---|
| FooDB ID | FDB016009 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00010537 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 3687785 |
|---|
| ChEBI ID | 167915 |
|---|
| PubChem Compound ID | 4490284 |
|---|
| Kegg Compound ID | C11647 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|