| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:02:42 UTC |
|---|
| Update Date | 2016-11-09 01:19:07 UTC |
|---|
| Accession Number | CHEM030636 |
|---|
| Identification |
|---|
| Common Name | Mozambioside |
|---|
| Class | Small Molecule |
|---|
| Description | Mozambioside is found in coffee and coffee products. Mozambioside is isolated from beans of arabica coffee (Coffea arabica) and wild coffee sp. (Coffea pseudozanguebariae). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
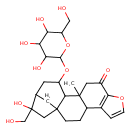
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C26H36O10 |
|---|
| Average Molecular Mass | 508.558 g/mol |
|---|
| Monoisotopic Mass | 508.231 g/mol |
|---|
| CAS Registry Number | 70717-95-2 |
|---|
| IUPAC Name | 17-hydroxy-17-(hydroxymethyl)-12-methyl-14-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-8-oxapentacyclo[14.2.1.0¹,¹³.0⁴,¹².0⁵,⁹]nonadeca-5(9),6-dien-10-one |
|---|
| Traditional Name | 17-hydroxy-17-(hydroxymethyl)-12-methyl-14-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-8-oxapentacyclo[14.2.1.0¹,¹³.0⁴,¹².0⁵,⁹]nonadeca-5(9),6-dien-10-one |
|---|
| SMILES | CC12CC(=O)C3=C(C=CO3)C1CCC13CC(CC(OC4OC(CO)C(O)C(O)C4O)C21)C(O)(CO)C3 |
|---|
| InChI Identifier | InChI=1S/C26H36O10/c1-24-8-15(29)21-13(3-5-34-21)14(24)2-4-25-7-12(26(33,10-25)11-28)6-16(22(24)25)35-23-20(32)19(31)18(30)17(9-27)36-23/h3,5,12,14,16-20,22-23,27-28,30-33H,2,4,6-11H2,1H3 |
|---|
| InChI Key | FNIBRRHOZLASGI-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as naphthofurans. Naphthofurans are compounds containing a furan ring fused to a naphthalene moiety. Furan is a 5 membered- ring aromatic ring with four carbon and one oxygen atoms. Naphthalene is a polycyclic aromatic hydrocarbon made up of two fused benzene rings. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Naphthofurans |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Naphthofurans |
|---|
| Alternative Parents | |
|---|
| Substituents | - Naphthofuran
- Hexose monosaccharide
- Glycosyl compound
- O-glycosyl compound
- Benzofuran
- Aryl ketone
- Aryl alkyl ketone
- Monosaccharide
- Oxane
- Heteroaromatic compound
- Furan
- Cyclic alcohol
- Tertiary alcohol
- Ketone
- Secondary alcohol
- Polyol
- Acetal
- Oxacycle
- Primary alcohol
- Organooxygen compound
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00bc-2200900000-aaac4fa9fd7ad8651011 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0079-2212029000-45e9af7dec92b345ad57 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-056v-0109320000-057da89ce5a309e5d9b2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004j-0109000000-8fbee9b24c4ff4350d2e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004j-1469000000-9d103ead1e2eaea81ea3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a6s-0209460000-b30c68176a0753e58bf4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00kb-1109200000-96e76d8178db95ac3b2b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-02ta-2009000000-739c3d4c2ef9b55a1da2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0000190000-3f897cefef790e5cfe16 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-3002690000-218488900e436cbced56 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4v-9001810000-72a4f8c9d384f324414e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0001190000-a7216bf9e3dcf530de04 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a6u-0027940000-b38093a9e214a9305708 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052s-9724800000-e474c18d6751cda88de9 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0037002 |
|---|
| FooDB ID | FDB015977 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 168885 |
|---|
| PubChem Compound ID | 73822377 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|