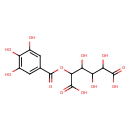| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:00:41 UTC |
|---|
| Update Date | 2016-11-09 01:19:06 UTC |
|---|
| Accession Number | CHEM030588 |
|---|
| Identification |
|---|
| Common Name | 2-Galloylgalactaric acid |
|---|
| Class | Small Molecule |
|---|
| Description | 2-O-Galloylgalactaric acid is found in fruits. 2-O-Galloylgalactaric acid is a constituent of the fruit of emblic (Phyllanthus emblica). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-O-Galloylgalactarate | Generator | | 2-O-Galloylmucic acid | HMDB | | 2,3,4-Trihydroxy-5-(3,4,5-trihydroxybenzoyloxy)hexanedioate | Generator | | 2-Galloylgalactarate | Generator |
|
|---|
| Chemical Formula | C13H14O12 |
|---|
| Average Molecular Mass | 362.243 g/mol |
|---|
| Monoisotopic Mass | 362.049 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 2,3,4-trihydroxy-5-(3,4,5-trihydroxybenzoyloxy)hexanedioic acid |
|---|
| Traditional Name | 2,3,4-trihydroxy-5-(3,4,5-trihydroxybenzoyloxy)hexanedioic acid |
|---|
| SMILES | OC(C(O)C(OC(=O)C1=CC(O)=C(O)C(O)=C1)C(O)=O)C(O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C13H14O12/c14-4-1-3(2-5(15)6(4)16)13(24)25-10(12(22)23)8(18)7(17)9(19)11(20)21/h1-2,7-10,14-19H,(H,20,21)(H,22,23) |
|---|
| InChI Key | KJWXZHRHCSMFSY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as glucuronic acid derivatives. Glucuronic acid derivatives are compounds containing a glucuronic acid moiety (or a derivative), which consists of a glucose moiety with the C6 carbon oxidized to a carboxylic acid. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Glucuronic acid derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Glucuronic acid or derivatives
- Galloyl ester
- Gallic acid or derivatives
- P-hydroxybenzoic acid alkyl ester
- M-hydroxybenzoic acid ester
- P-hydroxybenzoic acid ester
- Benzoate ester
- Benzenetriol
- Benzoic acid or derivatives
- Tricarboxylic acid or derivatives
- Pyrogallol derivative
- Benzoyl
- Phenol
- Beta-hydroxy acid
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Monosaccharide
- Hydroxy acid
- Benzenoid
- Alpha-hydroxy acid
- Monocyclic benzene moiety
- Secondary alcohol
- Carboxylic acid ester
- Polyol
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organic oxide
- Alcohol
- Carbonyl group
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0udi-8902000000-5a88bcee444dd9e8fd9c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-0udi-3901020000-4b3d4be072aabed502b8 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0gxt-1928000000-5651842f5d1362e1a2d7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0zi9-6952000000-1258300a0ab1d3b30acf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ufr-5900000000-4c744a5cc2a4f72d6675 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00or-7893000000-282d0b9d979c7e4ebf4b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0690-8960000000-748eefb1b21effff29b6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01di-3920000000-768c83e69086ca761c33 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0gvk-1598000000-1c64f7d1733071499b03 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-06ri-9540000000-7938213ac3d4955a373a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ufr-5900000000-2e78ff04222c412c0cbc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-046s-6494000000-1593879ef8ca97b45b91 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00n3-6970000000-b705df28236f3c9b313d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-056r-5900000000-040fcabe1c836d1f36b5 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036932 |
|---|
| FooDB ID | FDB015901 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35014322 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 75060826 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|