| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:00:07 UTC |
|---|
| Update Date | 2016-11-09 01:19:06 UTC |
|---|
| Accession Number | CHEM030576 |
|---|
| Identification |
|---|
| Common Name | (all-E)-Rubixanthin |
|---|
| Class | Small Molecule |
|---|
| Description | (all-E)-Rubixanthin is found in fruits. (all-E)-Rubixanthin is a constituent of ripe fruit of Rubus chamaemorus (cloudberry). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
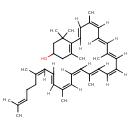
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C40H56O |
|---|
| Average Molecular Mass | 552.887 g/mol |
|---|
| Monoisotopic Mass | 552.433 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 4-[(1E,3Z,5E,7E,9Z,11E,13Z,15Z,17E,19E)-3,7,12,16,20,24-hexamethylpentacosa-1,3,5,7,9,11,13,15,17,19,23-undecaen-1-yl]-3,5,5-trimethylcyclohex-3-en-1-ol |
|---|
| Traditional Name | 4-[(1E,3Z,5E,7E,9Z,11E,13Z,15Z,17E,19E)-3,7,12,16,20,24-hexamethylpentacosa-1,3,5,7,9,11,13,15,17,19,23-undecaen-1-yl]-3,5,5-trimethylcyclohex-3-en-1-ol |
|---|
| SMILES | [H]\C(=C(/[H])\C(\[H])=C(/C)\C(\[H])=C(/[H])\C(\[H])=C(\C)C([H])=C([H])C1=C(C)CC(O)CC1(C)C)C([H])=C(C)C([H])=C([H])C(\[H])=C(\C)C([H])=C([H])C([H])=C(C)CCC=C(C)C |
|---|
| InChI Identifier | InChI=1S/C40H56O/c1-31(2)17-13-20-34(5)23-15-25-35(6)24-14-21-32(3)18-11-12-19-33(4)22-16-26-36(7)27-28-39-37(8)29-38(41)30-40(39,9)10/h11-12,14-19,21-28,38,41H,13,20,29-30H2,1-10H3/b12-11-,21-14-,22-16+,25-15+,28-27+,32-18+,33-19+,34-23-,35-24-,36-26- |
|---|
| InChI Key | ABTRFGSPYXCGMR-INLRYUFBSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as xanthophylls. These are carotenoids containing an oxygenated carotene backbone. Carotenes are characterized by the presence of two end-groups (mostly cyclohexene rings, but also cyclopentene rings or acyclic groups) linked by a long branched alkyl chain. Carotenes belonging form a subgroup of the carotenoids family. Xanthophylls arise by oxygenation of the carotene backbone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Tetraterpenoids |
|---|
| Direct Parent | Xanthophylls |
|---|
| Alternative Parents | |
|---|
| Substituents | - Xanthophyll
- Secondary alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000i-3000390000-981287e75d14b8e6cab4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0bt9-6100049000-7967ad61432f0b9c839f | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("(all-E)-Rubixanthin,1TMS,#1" TMS) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f79-0313090000-1c593709def3865bcb0f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000t-0649100000-f2a7db726b149bd94e9a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kb-2559200000-4dc6e92b0c4b9cd98b1c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0000090000-dac8fbbcd54bfe825425 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0000090000-229ec1e7491100bbf943 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-1546290000-cbc945a460fe0e67a925 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0104290000-78bc8eac3eeeae91238d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udj-0219160000-deb57f8b99639faa8e37 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052b-0239040000-2377c64c132866587681 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f6y-4117930000-c9d4425e3fa62d8e26ef | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0pc1-3113900000-b7bfbf01467932487b10 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-5019200000-0f4bcd494d739f7ab14a | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036921 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 74849519 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|