| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:59:42 UTC |
|---|
| Update Date | 2016-11-09 01:19:06 UTC |
|---|
| Accession Number | CHEM030568 |
|---|
| Identification |
|---|
| Common Name | Parasiloxanthin |
|---|
| Class | Small Molecule |
|---|
| Description | Parasiloxanthin is found in fishes. Parasiloxanthin is isolated from skin and fin of Japanese catfish (Parasilurus asotus). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
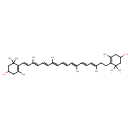
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 7',8'-dihydro-b,b-Carotene-3,3'-diol | HMDB | | 7,8-dihydro-beta,Beat-carotene-3,3'-diol | HMDB |
|
|---|
| Chemical Formula | C40H58O2 |
|---|
| Average Molecular Mass | 570.887 g/mol |
|---|
| Monoisotopic Mass | 570.444 g/mol |
|---|
| CAS Registry Number | 62994-48-3 |
|---|
| IUPAC Name | 4-[(3E,5E,7E,9E,11E,13E,15E,17E)-18-(4-hydroxy-2,6,6-trimethylcyclohex-1-en-1-yl)-3,7,12,16-tetramethyloctadeca-3,5,7,9,11,13,15,17-octaen-1-yl]-3,5,5-trimethylcyclohex-3-en-1-ol |
|---|
| Traditional Name | 4-[(3E,5E,7E,9E,11E,13E,15E,17E)-18-(4-hydroxy-2,6,6-trimethylcyclohex-1-en-1-yl)-3,7,12,16-tetramethyloctadeca-3,5,7,9,11,13,15,17-octaen-1-yl]-3,5,5-trimethylcyclohex-3-en-1-ol |
|---|
| SMILES | C\C(CCC1=C(C)CC(O)CC1(C)C)=C/C=C/C(/C)=C/C=C/C=C(\C)/C=C/C=C(\C)/C=C/C1=C(C)CC(O)CC1(C)C |
|---|
| InChI Identifier | InChI=1S/C40H58O2/c1-29(17-13-19-31(3)21-23-37-33(5)25-35(41)27-39(37,7)8)15-11-12-16-30(2)18-14-20-32(4)22-24-38-34(6)26-36(42)28-40(38,9)10/h11-21,23,35-36,41-42H,22,24-28H2,1-10H3/b12-11+,17-13+,18-14+,23-21+,29-15+,30-16+,31-19+,32-20+ |
|---|
| InChI Key | ZAYHYNGKERKFHJ-ZRVUQIMUSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as xanthophylls. These are carotenoids containing an oxygenated carotene backbone. Carotenes are characterized by the presence of two end-groups (mostly cyclohexene rings, but also cyclopentene rings or acyclic groups) linked by a long branched alkyl chain. Carotenes belonging form a subgroup of the carotenoids family. Xanthophylls arise by oxygenation of the carotene backbone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Tetraterpenoids |
|---|
| Direct Parent | Xanthophylls |
|---|
| Alternative Parents | |
|---|
| Substituents | - Xanthophyll
- Secondary alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0pvi-2400690000-1c63e14dea1df9ac8405 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-004i-2020339000-bf6a6fb81b9f220b42c0 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("Parasiloxanthin,1TMS,#1" TMS) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uk9-0101190000-45c9dce7caa208d68e29 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fr2-0529330000-f1926efded0b92e5e069 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f72-1449330000-52a36f72ace2547f2e6e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0000090000-4a41a91adb25acbb3819 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0000090000-38fad70e0862fbdd8c31 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udr-0363390000-6a6ae462dff02dffca88 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0fzi-0223970000-2bdfb6c4c8af72dd4e64 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01p9-0532940000-a479aef16b5fc06019c3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-003s-0697700000-980dea40a3e28092c8ed | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0000090000-28fc2d7258285ded8bbc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014r-0422190000-70aa76b3c6364e3cd032 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fb9-0219400000-d13ccc003e681544730f | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036915 |
|---|
| FooDB ID | FDB015880 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00022866 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35014313 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 87445983 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|