| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:56:07 UTC |
|---|
| Update Date | 2016-11-09 01:19:05 UTC |
|---|
| Accession Number | CHEM030498 |
|---|
| Identification |
|---|
| Common Name | Cembrene |
|---|
| Class | Small Molecule |
|---|
| Description | Constituent of oil of Pinus koraiensis (Korean pine)
Cembrene A, or sometimes neocembrene, is a natural monocyclic diterpene isolated from corals of the genus Nephthea. It is a colorless oil with a faint wax-like odor. Cembrene is found in fats and oils and herbs and spices. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
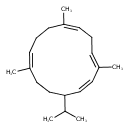
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+)-Cembrene | HMDB | | (+)-Thunbergen | HMDB | | (1S,2E,7E,11E)-2,4(18),7,11-Cembratraene | HMDB | | Cembrene a | HMDB | | Thumbelene | HMDB | | Thunbergen | HMDB | | Thunbergene | HMDB |
|
|---|
| Chemical Formula | C20H32 |
|---|
| Average Molecular Mass | 272.468 g/mol |
|---|
| Monoisotopic Mass | 272.250 g/mol |
|---|
| CAS Registry Number | 1898-13-1 |
|---|
| IUPAC Name | (1Z,3E,6Z,10Z)-3,7,11-trimethyl-14-(propan-2-yl)cyclotetradeca-1,3,6,10-tetraene |
|---|
| Traditional Name | (1Z,3E,6Z,10Z)-14-isopropyl-3,7,11-trimethylcyclotetradeca-1,3,6,10-tetraene |
|---|
| SMILES | CC(C)C1CC\C(C)=C/CC\C(C)=C/C\C=C(/C)\C=C/1 |
|---|
| InChI Identifier | InChI=1S/C20H32/c1-16(2)20-14-12-18(4)10-6-8-17(3)9-7-11-19(5)13-15-20/h8,10-12,14,16,20H,6-7,9,13,15H2,1-5H3/b14-12-,17-8-,18-10+,19-11- |
|---|
| InChI Key | DMHADBQKVWXPPM-ZRMRITCRSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as cembrane diterpenoids. These are diterpenoids with a structure based a cembrane skeleton, which is characterized by the presence of an isopropyl group at C-1 and by three symmetrically disposed methyl groups a the t C-4, -8 and -12 positions. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Diterpenoids |
|---|
| Direct Parent | Cembrane diterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cembrane diterpenoid
- Branched unsaturated hydrocarbon
- Cyclic olefin
- Unsaturated aliphatic hydrocarbon
- Unsaturated hydrocarbon
- Olefin
- Hydrocarbon
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004l-2090000000-bb2210617cf55eef5038 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0090000000-f085376fe769255a7e89 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00e9-0790000000-b8109296f529a9bc40a2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052r-1930000000-91a90a88f5481a4574d8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0090000000-52e1de275e919d42781d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0090000000-3734ab2300041c72bc6d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0aov-2290000000-cb45e61b0bf640153924 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036845 |
|---|
| FooDB ID | FDB015797 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Cembrene A |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131752064 |
|---|
| Kegg Compound ID | C11893 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Sawant SS, Sylvester PW, Avery MA, Desai P, Youssef DT, El Sayed KA: Bioactive rearranged and halogenated semisynthetic derivatives of the marine natural product sarcophine. J Nat Prod. 2004 Dec;67(12):2017-23. | | 2. Simons K, Toomre D: Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000 Oct;1(1):31-9. | | 3. Watson AD: Thematic review series: systems biology approaches to metabolic and cardiovascular disorders. Lipidomics: a global approach to lipid analysis in biological systems. J Lipid Res. 2006 Oct;47(10):2101-11. Epub 2006 Aug 10. | | 4. Sethi JK, Vidal-Puig AJ: Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res. 2007 Jun;48(6):1253-62. Epub 2007 Mar 20. | | 5. Lingwood D, Simons K: Lipid rafts as a membrane-organizing principle. Science. 2010 Jan 1;327(5961):46-50. doi: 10.1126/science.1174621. | | 6. Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC. | | 7. The lipid handbook with CD-ROM |
|
|---|