| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:55:06 UTC |
|---|
| Update Date | 2016-11-09 01:19:05 UTC |
|---|
| Accession Number | CHEM030475 |
|---|
| Identification |
|---|
| Common Name | Vitispirane |
|---|
| Class | Small Molecule |
|---|
| Description | Vitispirane is found in alcoholic beverages. Vitispirane is a constituent of the juice of wine grape (Vitis vinifera). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
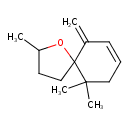
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2,10,10-Trimethyl-6-methylidene-1-oxaspiro[4.5]dec-7-ene | HMDB | | 6,9-Epoxy-3,5(13)-megastigmadiene | HMDB |
|
|---|
| Chemical Formula | C13H20O |
|---|
| Average Molecular Mass | 192.302 g/mol |
|---|
| Monoisotopic Mass | 192.151 g/mol |
|---|
| CAS Registry Number | 65416-59-3 |
|---|
| IUPAC Name | 2,10,10-trimethyl-6-methylidene-1-oxaspiro[4.5]dec-7-ene |
|---|
| Traditional Name | 2,10,10-trimethyl-6-methylidene-1-oxaspiro[4.5]dec-7-ene |
|---|
| SMILES | CC1CCC2(O1)C(=C)C=CCC2(C)C |
|---|
| InChI Identifier | InChI=1S/C13H20O/c1-10-6-5-8-12(3,4)13(10)9-7-11(2)14-13/h5-6,11H,1,7-9H2,2-4H3 |
|---|
| InChI Key | DUPDJVDPPBFBPL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tetrahydrofurans. These are heterocyclic compounds containing a saturated, aliphatic, five-membered ring where a carbon is replaced by an oxygen. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrahydrofurans |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Tetrahydrofurans |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tetrahydrofuran
- Oxacycle
- Ether
- Dialkyl ether
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-03gv-2900000000-2739a1eb76ca5414d126 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-1900000000-744c2618d9fcad65a6eb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kf-6900000000-eda4218a528077619319 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gb9-9100000000-0940e73441b1b0b4f7ee | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0900000000-593d1022019da0a50f12 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0900000000-e5d36e0a67335cf33415 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a70-3900000000-57f58811e3bfef9b8148 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0900000000-9cc3e24333b35d1cc976 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000l-5900000000-868d984a13f8e20c1337 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-9500000000-1e1f155e383051c8cb17 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0900000000-14436e4d9ff8d032db54 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0900000000-f2d4c7e0561b1f59c4d2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-02tl-4900000000-f45993d01a55623763bf | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036818 |
|---|
| FooDB ID | FDB015765 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00058171 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4953381 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 6450832 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | YMDB01413 |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|