| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:54:35 UTC |
|---|
| Update Date | 2016-11-09 01:19:04 UTC |
|---|
| Accession Number | CHEM030464 |
|---|
| Identification |
|---|
| Common Name | Aframodial |
|---|
| Class | Small Molecule |
|---|
| Description | Aframodial is found in ginger. Aframodial is a constituent of Zingiber officinale (ginger). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
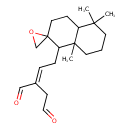
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 8b,17-Epoxy-12E-labdene-15,16-dial | HMDB | | 8beta,17-Epoxyl-12E-labdene-15,16-dial | HMDB | | Miogadial | HMDB | | ZT | HMDB | | 8,17-Epoxylabd-12-ene-15,16-dial | MeSH | | 8,17-Epoxylabd-12-ene-15,16-dial, (1R-(1alpha(e),2alpha,4abeta,8aalpha))-isomer | MeSH | | ZT-Dial | MeSH | | Aframodial | MeSH |
|
|---|
| Chemical Formula | C20H30O3 |
|---|
| Average Molecular Mass | 318.450 g/mol |
|---|
| Monoisotopic Mass | 318.219 g/mol |
|---|
| CAS Registry Number | 71641-23-1 |
|---|
| IUPAC Name | (2E)-2-(2-{5,5,8a-trimethyl-octahydro-1H-spiro[naphthalene-2,2'-oxirane]-1-yl}ethylidene)butanedial |
|---|
| Traditional Name | (2E)-2-(2-{5,5,8a-trimethyl-hexahydro-1H-spiro[naphthalene-2,2'-oxirane]-1-yl}ethylidene)butanedial |
|---|
| SMILES | CC1(C)CCCC2(C)C(C\C=C(/CC=O)C=O)C3(CO3)CCC12 |
|---|
| InChI Identifier | InChI=1S/C20H30O3/c1-18(2)9-4-10-19(3)16(18)7-11-20(14-23-20)17(19)6-5-15(13-22)8-12-21/h5,12-13,16-17H,4,6-11,14H2,1-3H3/b15-5+ |
|---|
| InChI Key | ZAWCPGMKVKTLKI-PJQLUOCWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as diterpenoids. These are terpene compounds formed by four isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Diterpenoids |
|---|
| Direct Parent | Diterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Labdane diterpenoid
- Diterpenoid
- Alpha,beta-unsaturated aldehyde
- Alpha-hydrogen aldehyde
- Enal
- Dialkyl ether
- Oxacycle
- Organoheterocyclic compound
- Oxirane
- Ether
- Hydrocarbon derivative
- Aldehyde
- Organic oxygen compound
- Organic oxide
- Carbonyl group
- Organooxygen compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000i-1492000000-200c965f9d8f3b227008 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-1059000000-ad9aba90d8d2eb5f383f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0005-8092000000-49b7479df99d1d286499 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fr5-9540000000-3f447be100f7d2d3b80c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0029000000-665115ad33e4a49ef13c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-5079000000-93e71efaaa76dccab567 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9040000000-e172af03159464d61857 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014r-0097000000-9840a2772ade2353d466 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0091000000-b52443d113ef3c761b41 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0aor-7190000000-bb8554313d6dff1f17c7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0019000000-17e99d8432551e2230cc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ff0-3796000000-f05daf0a146e6f086838 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-9412000000-7ce6838d77032402f7f9 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036806 |
|---|
| FooDB ID | FDB015752 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00022440 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35014255 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131752056 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|