| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:54:20 UTC |
|---|
| Update Date | 2016-11-09 01:19:04 UTC |
|---|
| Accession Number | CHEM030457 |
|---|
| Identification |
|---|
| Common Name | Cycloseychellene |
|---|
| Class | Small Molecule |
|---|
| Description | A member of the class of rotenones that consists of 1,2,12,12a-tetrahydrochromeno[3,4-b]furo[2,3-h]chromen-6(6aH)-one substituted at position 2 by a prop-1-en-2-yl group and at positions 8 and 9 by methoxy groups (the 2R,6aS,12aS-isomer). A non-systemic insecticide, it is the principal insecticidal constituent of derris (the dried rhizome and root of Derris elliptica). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
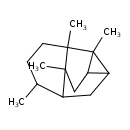
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (-)-cis-Rotenone | ChEBI | | (-)-Rotenone | ChEBI | | 5'beta-Rotenone | ChEBI | | [2R-(2alpha,6Aalpha,12aalpha)]-1,2,12,12a-tetrahydro-8,9-dimethoxy-2-(1-methylethenyl)[1]benzopyrano[3,4-b]furo[2,3-H][1]benzopyran-6(6ah)-one | ChEBI | | Barbasco | ChEBI | | Canex | ChEBI | | Dactinol | ChEBI | | Derris | ChEBI | | Noxfire | ChEBI | | Paraderil | ChEBI | | Tubatoxin | ChEBI | | 5'b-Rotenone | Generator | | 5'Β-rotenone | Generator | | [2R-(2a,6Aalpha,12aalpha)]-1,2,12,12a-tetrahydro-8,9-dimethoxy-2-(1-methylethenyl)[1]benzopyrano[3,4-b]furo[2,3-H][1]benzopyran-6(6ah)-one | Generator | | [2R-(2Α,6aalpha,12aalpha)]-1,2,12,12a-tetrahydro-8,9-dimethoxy-2-(1-methylethenyl)[1]benzopyrano[3,4-b]furo[2,3-H][1]benzopyran-6(6ah)-one | Generator | | 1,2,12,12a-Tetrahydro-8,9-dimethoxy-2-(1-methylethenyl)-[1]benzopyrano[3,4-b]furo[2,3-H][1]benzopyran-6(6ah)-one, 9ci | HMDB | | Derrin | HMDB | | Derris, jmaf | HMDB | | Dri-kil | HMDB | | Nicouline | HMDB | | Noxfish | HMDB | | Tubotoxin | HMDB |
|
|---|
| Chemical Formula | C15H24 |
|---|
| Average Molecular Mass | 204.351 g/mol |
|---|
| Monoisotopic Mass | 204.188 g/mol |
|---|
| CAS Registry Number | 52617-34-2 |
|---|
| IUPAC Name | 4,7,8,11-tetramethyltetracyclo[5.4.0.0³,⁵.0⁴,⁸]undecane |
|---|
| Traditional Name | 4,7,8,11-tetramethyltetracyclo[5.4.0.0³,⁵.0⁴,⁸]undecane |
|---|
| SMILES | CC1CCC2(C)C3(C)C4CC2(C)C1CC34 |
|---|
| InChI Identifier | InChI=1S/C15H24/c1-9-5-6-14(3)13(2)8-12-11(7-10(9)13)15(12,14)4/h9-12H,5-8H2,1-4H3 |
|---|
| InChI Key | XPWRIXBORAHMCD-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as rotenones. These are rotenoids with a structure based on a 6a,12a-dihydrochromeno[3,4-b]chromen-12(6H)-one skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Isoflavonoids |
|---|
| Sub Class | Rotenoids |
|---|
| Direct Parent | Rotenones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Rotenone or derivatives
- 8-prenylated isoflavanone
- Isoflavanone
- Isoflavan
- Chromone
- Chromane
- 1-benzopyran
- Benzopyran
- Coumaran
- Anisole
- Aryl alkyl ketone
- Aryl ketone
- Alkyl aryl ether
- Benzenoid
- Ketone
- Ether
- Oxacycle
- Organoheterocyclic compound
- Organic oxygen compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000i-3900000000-2b9c1013929c318bce4a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0090000000-98b8e4eab8cd946d233c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-1490000000-7a725e3ea16e1b28ba40 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-06te-3900000000-d72662a16adb4f83c184 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-d0fe5ed0bbcfa60c303c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0090000000-829ffa2a496cae88f4ca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fe0-1920000000-f32b62ce5cffd0ea4d7d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0090000000-c084f3d998cc56a199d6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4j-0590000000-e270a4e16b089b4c94c0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052b-0960000000-75a6df425fb300f2e2b2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-7ccf03fa1149a1e9f55f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0090000000-7ccf03fa1149a1e9f55f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-0090000000-7ccf03fa1149a1e9f55f | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB11457 |
|---|
| HMDB ID | HMDB0034436 |
|---|
| FooDB ID | FDB012837 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00002568 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | CPD-5741 |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Rotenone |
|---|
| Chemspider ID | 6500 |
|---|
| ChEBI ID | 28201 |
|---|
| PubChem Compound ID | 6758 |
|---|
| Kegg Compound ID | C07593 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|