| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:54:06 UTC |
|---|
| Update Date | 2016-11-09 01:19:04 UTC |
|---|
| Accession Number | CHEM030451 |
|---|
| Identification |
|---|
| Common Name | gamma-Caryophyllene |
|---|
| Class | Small Molecule |
|---|
| Description | A beta-caryophyllene in which the stereocentre adjacent to the exocyclic double bond has S configuration while the remaining stereocentre has R configuration. It is the most commonly occurring form of beta-caryophyllene, occurring in many essential oils, particularly oil of cloves. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
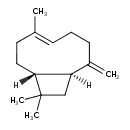
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (-)-(e)-beta-Caryophyllene | ChEBI | | (e)-beta-Caryophyllene | ChEBI | | Caryophyllene | ChEBI | | trans-(1R,9S)-4,11,11-Trimethyl-8-methylenebicyclo[7.2.0]undec-4-ene | ChEBI | | trans-Caryophyllene | ChEBI | | (-)-beta-Caryophyllene | Kegg | | (-)-(e)-b-Caryophyllene | Generator | | (-)-(e)-Β-caryophyllene | Generator | | (e)-b-Caryophyllene | Generator | | (e)-Β-caryophyllene | Generator | | (-)-b-Caryophyllene | Generator | | (-)-Β-caryophyllene | Generator | | b-Caryophyllene | Generator | | Β-caryophyllene | Generator | | Caryophyllene, (S-(r*,4Z,9S*))-isomer | HMDB | | Caryophyllene, (R-(r*,4E,9S))-isomer | HMDB | | Caryophyllene, (r*,4Z,9S*)-(+-)-isomer | HMDB | | Caryophyllene, (R-(r*,4Z,9S*))-isomer | HMDB | | Caryophyllene, (S-(r*,4E,9S*))-isomer | HMDB | | (-)-(e)-Caryophyllene | HMDB | | (-)-e-Caryophyllene | HMDB | | (-)-Caryophyllene | HMDB | | (-)-trans-Caryophyllene | HMDB | | (e)-Caryophyllene | HMDB | | Caryophyllene b | HMDB | | NSC 11906 | HMDB | | trans-Β-caryophyllene | HMDB | | trans-beta-Caryophyllene | HMDB | | Β-caryophyllen | HMDB | | beta-Caryophyllen | HMDB | | beta-Caryophyllene | HMDB, ChEBI | | l-Caryophyllene | PhytoBank | | beta-Caryophellene | PhytoBank | | β-Caryophellene | PhytoBank |
|
|---|
| Chemical Formula | C15H24 |
|---|
| Average Molecular Mass | 204.351 g/mol |
|---|
| Monoisotopic Mass | 204.188 g/mol |
|---|
| CAS Registry Number | 118-65-0 |
|---|
| IUPAC Name | (1R,4E,9S)-4,11,11-trimethyl-8-methylidenebicyclo[7.2.0]undec-4-ene |
|---|
| Traditional Name | caryophyllene |
|---|
| SMILES | C\C1=C\CCC(=C)C2CC(C)(C)C2CC1 |
|---|
| InChI Identifier | InChI=1S/C15H24/c1-11-6-5-7-12(2)13-10-15(3,4)14(13)9-8-11/h6,13-14H,2,5,7-10H2,1,3-4H3/b11-6- |
|---|
| InChI Key | NPNUFJAVOOONJE-WDZFZDKYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sesquiterpenoids. These are terpenes with three consecutive isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesquiterpenoids |
|---|
| Direct Parent | Sesquiterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Caryophyllane sesquiterpenoid
- Sesquiterpenoid
- Branched unsaturated hydrocarbon
- Polycyclic hydrocarbon
- Cyclic olefin
- Unsaturated aliphatic hydrocarbon
- Unsaturated hydrocarbon
- Olefin
- Hydrocarbon
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-Q (Non-derivatized) | splash10-0006-9600000000-f461c075128fda03909d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-2900000000-666e65f6b538d4a0eeea | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0290000000-de2a6b536bb38d3c2951 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-3950000000-6dc317c917393691dfef | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f7a-6900000000-5f5ee8e9f24e0511e186 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-d6c31402204fa765cbc0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0290000000-48307d26c19d0c84ce74 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002r-2900000000-741197d2390e8d4bc104 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-7ccf03fa1149a1e9f55f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0090000000-7ccf03fa1149a1e9f55f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-0980000000-9f708eefb54c5860f003 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0190000000-18da8cef0774773a4664 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4j-7930000000-0de14671750170215e53 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4m-9400000000-c0fc2f9446fefc882918 | Spectrum | | MS | Mass Spectrum (Electron Ionization) | splash10-0006-9600000000-0eeaa207a8899311023d | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036792 |
|---|
| FooDB ID | FDB097248 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00003110 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | CPD-8230 |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Caryophyllene |
|---|
| Chemspider ID | 4444848 |
|---|
| ChEBI ID | 10357 |
|---|
| PubChem Compound ID | 5281515 |
|---|
| Kegg Compound ID | C09629 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. https://www.ncbi.nlm.nih.gov/pubmed/?term=12409018 | | 2. https://www.ncbi.nlm.nih.gov/pubmed/?term=18296628 | | 3. https://www.ncbi.nlm.nih.gov/pubmed/?term=18574142 | | 4. https://www.ncbi.nlm.nih.gov/pubmed/?term=20015227 | | 5. https://www.ncbi.nlm.nih.gov/pubmed/?term=20398787 | | 6. https://www.ncbi.nlm.nih.gov/pubmed/?term=20433083 | | 7. https://www.ncbi.nlm.nih.gov/pubmed/?term=21366052 | | 8. https://www.ncbi.nlm.nih.gov/pubmed/?term=21425686 | | 9. https://www.ncbi.nlm.nih.gov/pubmed/?term=21941920 | | 10. https://www.ncbi.nlm.nih.gov/pubmed/?term=27871898 | | 11. https://www.ncbi.nlm.nih.gov/pubmed/?term=30281175 | | 12. Karim H, Kamel M, Mouna BT, Thouraya C, Brahim M: Essential oil composition of Hypericum triquetrifolium Turra. aerial parts. Ital J Biochem. 2007 Mar;56(1):40-6. | | 13. Luo J, Guo X, Feng Y: [Constituents analysis on volatile oil of Pogostemon cablin from different collection time cultivated in Hainan]. Zhong Yao Cai. 2002 Jan;25(1):21-3. | | 14. Luo J, Feng Y, Guo X, Li X: [GC-MS analysis of volatile oil of herba Pogostemonis collected from Gaoyao county]. Zhong Yao Cai. 1999 Jan;22(1):25-8. | | 15. Nascimento JC, Barbosa LC, Paula VF, David JM, Fontana R, Silva LA, Franca RS: Chemical composition and antimicrobial activity of essential oils of Ocimum canum Sims. and Ocimum selloi Benth. An Acad Bras Cienc. 2011 Sep;83(3):787-99. | | 16. Bhatia SP, Letizia CS, Api AM: Fragrance material review on beta-caryophyllene alcohol. Food Chem Toxicol. 2008 Nov;46 Suppl 11:S95-6. doi: 10.1016/j.fct.2008.06.030. Epub 2008 Jul 1. | | 17. Simons K, Toomre D: Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000 Oct;1(1):31-9. | | 18. Watson AD: Thematic review series: systems biology approaches to metabolic and cardiovascular disorders. Lipidomics: a global approach to lipid analysis in biological systems. J Lipid Res. 2006 Oct;47(10):2101-11. Epub 2006 Aug 10. | | 19. Sethi JK, Vidal-Puig AJ: Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res. 2007 Jun;48(6):1253-62. Epub 2007 Mar 20. | | 20. Lingwood D, Simons K: Lipid rafts as a membrane-organizing principle. Science. 2010 Jan 1;327(5961):46-50. doi: 10.1126/science.1174621. | | 21. Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC. | | 22. The lipid handbook with CD-ROM | | 23. Wikipedia: http://en.wikipedia.org/wiki/(-)-beta-caryophyllene_synthase | | 24. Wikipedia: http://en.wikipedia.org/wiki/(+)-beta-caryophyllene_synthase | | 25. Wikipedia: http://en.wikipedia.org/wiki/(E)-2-epi-beta-caryophyllene_synthase | | 26. MetaCyc: beta-caryophyllene biosynthesis: http://metacyc.org/META/NEW-IMAGE?type=PATHWAY&object=PWY-6275 | | 27. UniProt I6RAQ6 : (-)-beta-caryophyllene synthase: http://www.uniprot.org/uniprot/I6RAQ6 | | 28. UniProt J7LJN5 : Beta-caryophyllene synthase: http://www.uniprot.org/uniprot/J7LJN5 | | 29. UniProt Q8SA63 : Beta-caryophyllene synthase: http://www.uniprot.org/uniprot/Q8SA63 |
|
|---|