| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:53:35 UTC |
|---|
| Update Date | 2016-11-09 01:19:04 UTC |
|---|
| Accession Number | CHEM030438 |
|---|
| Identification |
|---|
| Common Name | Diosbulbin B |
|---|
| Class | Small Molecule |
|---|
| Description | Constituent of Dioscorea bulbifera (air potato). Diosbulbin B is found in root vegetables. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
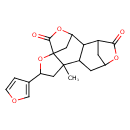
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 8b,12A:15,16-diepoxy-19-nor-13(16),14-clerodadiene-17,6b:18,2a-diolide | HMDB | | Diosbulbin b | MeSH |
|
|---|
| Chemical Formula | C19H20O6 |
|---|
| Average Molecular Mass | 344.359 g/mol |
|---|
| Monoisotopic Mass | 344.126 g/mol |
|---|
| CAS Registry Number | 20086-06-0 |
|---|
| IUPAC Name | 3-(furan-3-yl)-5-methyl-2,9,14-trioxapentacyclo[11.2.1.1⁸,¹¹.0¹,⁵.0⁶,¹²]heptadecane-10,15-dione |
|---|
| Traditional Name | 3-(furan-3-yl)-5-methyl-2,9,14-trioxapentacyclo[11.2.1.1⁸,¹¹.0¹,⁵.0⁶,¹²]heptadecane-10,15-dione |
|---|
| SMILES | CC12CC(OC11CC(OC1=O)C1C3CC(CC21)OC3=O)C1=COC=C1 |
|---|
| InChI Identifier | InChI=1S/C19H20O6/c1-18-6-13(9-2-3-22-8-9)25-19(18)7-14(24-17(19)21)15-11-4-10(5-12(15)18)23-16(11)20/h2-3,8,10-15H,4-7H2,1H3 |
|---|
| InChI Key | QEANLIISUSNNDX-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as naphthofurans. Naphthofurans are compounds containing a furan ring fused to a naphthalene moiety. Furan is a 5 membered- ring aromatic ring with four carbon and one oxygen atoms. Naphthalene is a polycyclic aromatic hydrocarbon made up of two fused benzene rings. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Naphthofurans |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Naphthofurans |
|---|
| Alternative Parents | |
|---|
| Substituents | - Naphthofuran
- Caprolactone
- Oxepane
- Dicarboxylic acid or derivatives
- Gamma butyrolactone
- Heteroaromatic compound
- Furan
- Tetrahydrofuran
- Carboxylic acid ester
- Lactone
- Carboxylic acid derivative
- Dialkyl ether
- Ether
- Oxacycle
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Carbonyl group
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00kb-9275000000-2ef4280e82f903237ba5 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0039000000-a6572b8a79f2176ce9f3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fdk-1395000000-1f676a5457ebef2fc9fa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00dj-8921000000-a6ae5cbdb602fd749d46 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0007-0059000000-b90a5764839968f560fe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014m-5089000000-32a45f1eaa1c12b859c4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-2290000000-c1477989aed404305441 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0009000000-76730524e176cf7619b3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0059000000-5e3cd9a0855916ba5486 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000t-4292000000-d28ccbac0adf55777beb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0009000000-48250ff27042a4c0a8fa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00kf-0019000000-5b8c40239e60b0777cdf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kb-9174000000-30066b55c0bb525a25df | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036777 |
|---|
| FooDB ID | FDB015719 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 21136500 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 15559043 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|