| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:53:12 UTC |
|---|
| Update Date | 2016-11-09 01:19:04 UTC |
|---|
| Accession Number | CHEM030428 |
|---|
| Identification |
|---|
| Common Name | Zederone |
|---|
| Class | Small Molecule |
|---|
| Description | Zederone is a constituent of the rhizome of Curcuma zedoaria (zedoary). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
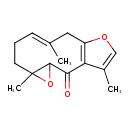
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Cyanidin 3-(6-O-p-coumarylglucoside) | HMDB | | Cyanidin 3-(6-p-coumaroylglucoside) | HMDB | | Cyanidin 3-O-(6''-p-coumaroyl-glucoside) | HMDB | | Zederone | MeSH |
|
|---|
| Chemical Formula | C15H18O3 |
|---|
| Average Molecular Mass | 246.302 g/mol |
|---|
| Monoisotopic Mass | 246.126 g/mol |
|---|
| CAS Registry Number | 7727-79-9 |
|---|
| IUPAC Name | (8E)-5,9,14-trimethyl-4,12-dioxatricyclo[9.3.0.0³,⁵]tetradeca-1(11),8,13-trien-2-one |
|---|
| Traditional Name | (8E)-5,9,14-trimethyl-4,12-dioxatricyclo[9.3.0.0³,⁵]tetradeca-1(11),8,13-trien-2-one |
|---|
| SMILES | CC1=COC2=C1C(=O)C1OC1(C)CC\C=C(C)\C2 |
|---|
| InChI Identifier | InChI=1S/C15H18O3/c1-9-5-4-6-15(3)14(18-15)13(16)12-10(2)8-17-11(12)7-9/h5,8,14H,4,6-7H2,1-3H3/b9-5+ |
|---|
| InChI Key | CVIVANCKIBYAOP-WEVVVXLNSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as germacrane sesquiterpenoids. These are sesquiterpenoids having the germacrane skeleton, with a structure characterized by a cyclodecane ring substituted with an isopropyl and two methyl groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesquiterpenoids |
|---|
| Direct Parent | Germacrane sesquiterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Germacrane sesquiterpenoid
- Aryl ketone
- Aryl alkyl ketone
- Furan
- Heteroaromatic compound
- Ketone
- Organoheterocyclic compound
- Ether
- Oxirane
- Oxacycle
- Dialkyl ether
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-003s-3090000000-3440ed8172a145d43063 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0090000000-dfb179f736c51fef9ec3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-3290000000-57b0b09eea5a72942747 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-e8c50acf616f80050e2d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-5a1ce587affb3e85371d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0090000000-84fa1adc596be1c286c4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a5a-7910000000-4ea27ab7f39c5a20d971 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0090000000-7216d1413b7714a094f6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-002b-0090000000-bc563c15d1b661e0b96f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-0090000000-9b61bf21c39cb64de8bf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-851e47b490f6973ef9e5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0090000000-ed1ef60c1b631f7b92d7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004l-0090000000-f03d8d8692c0f259ff03 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036767 |
|---|
| FooDB ID | FDB015708 |
|---|
| Phenol Explorer ID | 68 |
|---|
| KNApSAcK ID | C00006800 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Phenylacetaldehyde |
|---|
| Chemspider ID | 24808209 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 11492496 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|