| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:53:08 UTC |
|---|
| Update Date | 2016-11-09 01:19:04 UTC |
|---|
| Accession Number | CHEM030426 |
|---|
| Identification |
|---|
| Common Name | Romucosine D |
|---|
| Class | Small Molecule |
|---|
| Description | Alkaloid from Rollinia mucosa (biriba). Romucosine D is found in alcoholic beverages and fruits. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
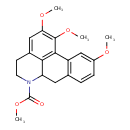
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+)-Romucosine D | HMDB | | Methyl 4,15,16-trimethoxy-10-azatetracyclo[7.7.1.0²,⁷.0¹³,¹⁷]heptadeca-1(17),2(7),3,5,13,15-hexaene-10-carboxylic acid | Generator |
|
|---|
| Chemical Formula | C21H23NO5 |
|---|
| Average Molecular Mass | 369.411 g/mol |
|---|
| Monoisotopic Mass | 369.158 g/mol |
|---|
| CAS Registry Number | 356048-26-5 |
|---|
| IUPAC Name | methyl 4,15,16-trimethoxy-10-azatetracyclo[7.7.1.0²,⁷.0¹³,¹⁷]heptadeca-1(17),2(7),3,5,13,15-hexaene-10-carboxylate |
|---|
| Traditional Name | methyl 4,15,16-trimethoxy-10-azatetracyclo[7.7.1.0²,⁷.0¹³,¹⁷]heptadeca-1(17),2(7),3,5,13,15-hexaene-10-carboxylate |
|---|
| SMILES | COC(=O)N1CCC2=CC(OC)=C(OC)C3=C2C1CC1=C3C=C(OC)C=C1 |
|---|
| InChI Identifier | InChI=1S/C21H23NO5/c1-24-14-6-5-12-9-16-18-13(7-8-22(16)21(23)27-4)10-17(25-2)20(26-3)19(18)15(12)11-14/h5-6,10-11,16H,7-9H2,1-4H3 |
|---|
| InChI Key | GIUSYZFFMYWEAY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as aporphines. These are quinoline alkaloids containing the dibenzo[de,g]quinoline ring system or a dehydrogenated derivative thereof. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Alkaloids and derivatives |
|---|
| Class | Aporphines |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Aporphines |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | Not Available |
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0ug0-0029000000-e2d1965e4275da374c40 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0009000000-2c058335ded230264e6b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0019000000-cc289bba3da7f7cf2bb4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0h0u-0092000000-86f5146154ac31694b1c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014r-0009000000-4e7f8b64336181504132 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0009000000-77f50113f86cd1c525fe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-2049000000-5998ebc75370b77a9006 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0009000000-7fb18f76427dfc094ae4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-0092000000-aea3583735a1b8afde1a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-4094000000-3d18402e2a6ef6ac6f04 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0009000000-cb20214e55840252e9f1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01w1-0029000000-a65dafd6d1feb613c33d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-02aj-0094000000-885a254209ff2329714c | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036765 |
|---|
| FooDB ID | FDB015706 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00037748 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 85102142 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|