| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:52:45 UTC |
|---|
| Update Date | 2016-11-09 01:19:04 UTC |
|---|
| Accession Number | CHEM030416 |
|---|
| Identification |
|---|
| Common Name | ent-16a-Hydro-6a,7a,17-trihydroxy-19-kauranoic acid |
|---|
| Class | Small Molecule |
|---|
| Description | Constituent of Acorus calamus (sweet flag). Acorusnol is found in herbs and spices and root vegetables. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
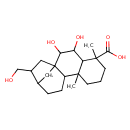
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2,3-Dihydroxy-14-(hydroxymethyl)-5,9-dimethyltetracyclo[11.2.1.0¹,¹⁰.0⁴,⁹]hexadecane-5-carboxylate | Generator | | (ent-6a,7a,16AlphaH)-6,7,17-trihydroxy-19-kauranoate | Generator | | (ent-6a,7a,16AlphaH)-6,7,17-trihydroxy-19-kauranoic acid | Generator | | (ent-6alpha,7alpha,16AlphaH)-6,7,17-trihydroxy-19-kauranoate | Generator | | (ent-6Α,7α,16alphah)-6,7,17-trihydroxy-19-kauranoate | Generator | | (ent-6Α,7α,16alphah)-6,7,17-trihydroxy-19-kauranoic acid | Generator |
|
|---|
| Chemical Formula | C20H32O5 |
|---|
| Average Molecular Mass | 352.465 g/mol |
|---|
| Monoisotopic Mass | 352.225 g/mol |
|---|
| CAS Registry Number | 56064-72-3 |
|---|
| IUPAC Name | 2,3-dihydroxy-14-(hydroxymethyl)-5,9-dimethyltetracyclo[11.2.1.0¹,¹⁰.0⁴,⁹]hexadecane-5-carboxylic acid |
|---|
| Traditional Name | 2,3-dihydroxy-14-(hydroxymethyl)-5,9-dimethyltetracyclo[11.2.1.0¹,¹⁰.0⁴,⁹]hexadecane-5-carboxylic acid |
|---|
| SMILES | CC12CCCC(C)(C1C(O)C(O)C13CC(CO)C(C1)CCC23)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C20H32O5/c1-18-6-3-7-19(2,17(24)25)15(18)14(22)16(23)20-8-11(4-5-13(18)20)12(9-20)10-21/h11-16,21-23H,3-10H2,1-2H3,(H,24,25) |
|---|
| InChI Key | FQXFNQBHZJYODR-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as eudesmane, isoeudesmane or cycloeudesmane sesquiterpenoids. These are sesquiterpenoids with a structure based on the eudesmane skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesquiterpenoids |
|---|
| Direct Parent | Eudesmane, isoeudesmane or cycloeudesmane sesquiterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Eudesmane, isoeudesmane or cycloeudesmane sesquiterpenoid
- Secondary alcohol
- Ketone
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-008i-2948000000-cfa61bd9c75f632da1c6 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-004i-1011249000-dce997746b7dc7b06d5c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f79-0019000000-228ac8907bad784f002c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kr-0149000000-d812897831c56df9e301 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-3294000000-3b26920672c3e20dcb1e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0019000000-3700a0981b32106493cd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0zpr-0049000000-f14f451861e48bb96f0f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-2094000000-a9d9a89a82b5513f1fe1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0009000000-380bcdac41775c30cac2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0029000000-bd410a1d72124635fcd9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0uk9-0049000000-faa268b52710d38766b0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0009000000-04fec15919a137f4a57e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-1009-1079000000-647b0af7d7410a8a5f16 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pvr-8579000000-d98eb42e551b9f4c41e0 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030920 |
|---|
| FooDB ID | FDB002886 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131751098 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|