| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:52:30 UTC |
|---|
| Update Date | 2016-11-09 01:19:04 UTC |
|---|
| Accession Number | CHEM030409 |
|---|
| Identification |
|---|
| Common Name | Momilactone B |
|---|
| Class | Small Molecule |
|---|
| Description | Constituent of Oryza sativa (rice)
Momilactone B is an allelopathic agent produced from the roots of rice (Oryza sativa L.) (100 mg from 200 kg dry rice husk). It has been shown to be produced in high concentrations by the roots of rice seedlings. The production of momilactone B has also been induced in response to infection by blast fungus (Pyricularia oryzae) or irradiated with UV light. More recently is has been shown to be a potential chemotherapeutic agent against human colon cancer.; The second step is the cyclization of syn-CDP to 9?-pimara-7,15-diene. This step is initiated by the elimination of the diphosphate group, a type A cyclization. The genes encoding for the type A cyclase were found by Otomo et al. in 2004. It is suggested that OsKS4, located on chromosome 4 (14.3cM) is one of the genes responsible for phytoalexin biosynthesis. After UV-radiation, OsKS4 mRNA levels rise drastically in response to the attack. Momilactone B is found in cereals and cereal products and rice. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
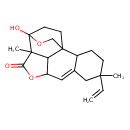
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3b,20-Epoxy-3a-hydroxy-7,15-pimaradien-19,6b-olide | HMDB | | Momilacton b | HMDB | | Momilactone b | MeSH |
|
|---|
| Chemical Formula | C20H26O4 |
|---|
| Average Molecular Mass | 330.418 g/mol |
|---|
| Monoisotopic Mass | 330.183 g/mol |
|---|
| CAS Registry Number | 51415-08-8 |
|---|
| IUPAC Name | 5-ethenyl-13-hydroxy-5,12-dimethyl-10,14-dioxapentacyclo[11.2.2.1¹,⁹.0²,⁷.0¹²,¹⁸]octadec-7-en-11-one |
|---|
| Traditional Name | 5-ethenyl-13-hydroxy-5,12-dimethyl-10,14-dioxapentacyclo[11.2.2.1¹,⁹.0²,⁷.0¹²,¹⁸]octadec-7-en-11-one |
|---|
| SMILES | CC12C3C(OC1=O)C=C1CC(C)(CCC1C31CCC2(O)OC1)C=C |
|---|
| InChI Identifier | InChI=1S/C20H26O4/c1-4-17(2)6-5-13-12(10-17)9-14-15-18(3,16(21)24-14)20(22)8-7-19(13,15)11-23-20/h4,9,13-15,22H,1,5-8,10-11H2,2-3H3 |
|---|
| InChI Key | SONPFFIKLYCKOY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as naphthopyrans. Naphthopyrans are compounds containing a pyran ring fused to a naphthalene moiety. Furan is a 6 membered-ring non-aromatic ring with five carbon and one oxygen atoms. Naphthalene is a polycyclic aromatic hydrocarbon made up of two fused benzene rings. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Naphthopyrans |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Naphthopyrans |
|---|
| Alternative Parents | |
|---|
| Substituents | - Naphthopyran
- Naphthofuran
- Naphthalene
- Furopyran
- Gamma butyrolactone
- Oxane
- Pyran
- Furan
- Cyclic alcohol
- Tetrahydrofuran
- Lactone
- Hemiacetal
- Carboxylic acid ester
- Oxacycle
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-014r-7594000000-262e9a486816a49966ed | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-000i-9204000000-01083810d31bad04f17a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0029000000-efd9dd6471fec372d203 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00lr-5259000000-4d21f007a0c800a54780 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gb9-9441000000-d1bb689b6f85a17a1415 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0009000000-71a231edd4c086920a1c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0019000000-dcaf394ed89d9e3d9fba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00li-0590000000-28abb07c252c401a32ac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0009000000-00a7d712e9bc4f24f659 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001j-0095000000-c0ceddbd2548e575cfc3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001a-2960000000-51d1f38d076c94076b61 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0009000000-f547833d1942b8c5fe90 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0009000000-f547833d1942b8c5fe90 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01t9-1059000000-487b3e63f89e0fc52e7a | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036749 |
|---|
| FooDB ID | FDB015687 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00000260 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Momilactone B |
|---|
| Chemspider ID | 24785551 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 73018629 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|