| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:52:28 UTC |
|---|
| Update Date | 2016-11-09 01:19:04 UTC |
|---|
| Accession Number | CHEM030408 |
|---|
| Identification |
|---|
| Common Name | Momilactone A |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
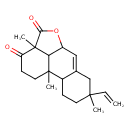
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3-oxo-9beta-Pimara-7,15-dien-19,6beta-olide | ChEBI | | Momilacton a | ChEBI | | 3-oxo-9b-Pimara-7,15-dien-19,6b-olide | Generator | | 3-oxo-9Β-pimara-7,15-dien-19,6β-olide | Generator | | 3-oxo-7,15-Pimaradien-19,6-olide | HMDB | | 6beta,18-Epoxy-9beta-pimara-7,15-diene-3,18-dione | HMDB |
|
|---|
| Chemical Formula | C20H26O3 |
|---|
| Average Molecular Mass | 314.419 g/mol |
|---|
| Monoisotopic Mass | 314.188 g/mol |
|---|
| CAS Registry Number | 51415-07-7 |
|---|
| IUPAC Name | 5-ethenyl-1,5,12-trimethyl-10-oxatetracyclo[7.6.1.0²,⁷.0¹²,¹⁶]hexadec-7-ene-11,13-dione |
|---|
| Traditional Name | 5-ethenyl-1,5,12-trimethyl-10-oxatetracyclo[7.6.1.0²,⁷.0¹²,¹⁶]hexadec-7-ene-11,13-dione |
|---|
| SMILES | CC12C3C(OC1=O)C=C1CC(C)(CCC1C3(C)CCC2=O)C=C |
|---|
| InChI Identifier | InChI=1S/C20H26O3/c1-5-18(2)8-6-13-12(11-18)10-14-16-19(13,3)9-7-15(21)20(16,4)17(22)23-14/h5,10,13-14,16H,1,6-9,11H2,2-4H3 |
|---|
| InChI Key | MPHXYQVSOFGNEN-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as diterpene lactones. These are diterpenoids containing a lactone moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Terpene lactones |
|---|
| Direct Parent | Diterpene lactones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Diterpene lactone
- Diterpenoid
- Pimarane diterpenoid
- Naphthofuran
- Gamma butyrolactone
- Tetrahydrofuran
- Lactone
- Ketone
- Carboxylic acid ester
- Organoheterocyclic compound
- Monocarboxylic acid or derivatives
- Oxacycle
- Carboxylic acid derivative
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05a2-3190000000-7ebc7a8b9b8716c7ff14 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0079000000-6d8ba4ae164a5fffe329 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-015a-4192000000-e92037d4ace346f79ea8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gb9-9240000000-459afc60580819fada6f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0029000000-378cce256f321f336f77 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0049000000-d92441dd87b762e99150 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0gb9-1090000000-0207db56e84ac9b5b9ca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0049000000-f693706d7f69133ae0d0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0091000000-2f01c12148a83263de15 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00m0-3970000000-137e436b0061865959de | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0009000000-d8638daf32922c227464 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0009000000-1a1a2f44fcb3d9289998 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03dj-0093000000-f7a924220f7d0b43802a | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00000259 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 142795 |
|---|
| ChEBI ID | 49191 |
|---|
| PubChem Compound ID | 162644 |
|---|
| Kegg Compound ID | C18015 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|