| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:50:01 UTC |
|---|
| Update Date | 2016-11-09 01:19:03 UTC |
|---|
| Accession Number | CHEM030342 |
|---|
| Identification |
|---|
| Common Name | 5,7alpha-Dihydro-1,4,4,7a-tetramethyl-4H-indene |
|---|
| Class | Small Molecule |
|---|
| Description | 5,7alpha-Dihydro-1,4,4,7a-tetramethyl-4H-indene is found in fruits. 5,7alpha-Dihydro-1,4,4,7a-tetramethyl-4H-indene is a constituent of quince fruit flavour (Cydonia oblonga). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
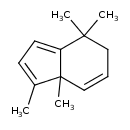
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 5,7a-Dihydro-1,4,4,7a-tetramethyl-4H-indene | Generator | | 5,7Α-dihydro-1,4,4,7a-tetramethyl-4H-indene | Generator | | 2,2,6,7-tetramethylbicyclo[4.3.0]Nona-1(9),4,7-triene | HMDB |
|
|---|
| Chemical Formula | C13H18 |
|---|
| Average Molecular Mass | 174.282 g/mol |
|---|
| Monoisotopic Mass | 174.141 g/mol |
|---|
| CAS Registry Number | 99901-21-0 |
|---|
| IUPAC Name | 3,3a,7,7-tetramethyl-6,7-dihydro-3aH-indene |
|---|
| Traditional Name | 1,4,4,7a-tetramethyl-5H-indene |
|---|
| SMILES | CC1=CC=C2C1(C)C=CCC2(C)C |
|---|
| InChI Identifier | InChI=1S/C13H18/c1-10-6-7-11-12(2,3)8-5-9-13(10,11)4/h5-7,9H,8H2,1-4H3 |
|---|
| InChI Key | XOFDOXJFXCEFDE-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as branched unsaturated hydrocarbons. These are hydrocarbons that contains one or more unsaturated carbon atoms, and an aliphatic branch. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Hydrocarbons |
|---|
| Class | Unsaturated hydrocarbons |
|---|
| Sub Class | Branched unsaturated hydrocarbons |
|---|
| Direct Parent | Branched unsaturated hydrocarbons |
|---|
| Alternative Parents | |
|---|
| Substituents | - Branched unsaturated hydrocarbon
- Polycyclic hydrocarbon
- Cyclic olefin
- Unsaturated aliphatic hydrocarbon
- Olefin
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-053r-1900000000-40fd9d68b8176c61852f | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0900000000-4abfb9f37007fcbd7a0c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-3900000000-c25881bface2981e4b7f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-100r-9400000000-8f537bd24eaf5a0813c1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0900000000-56fe880b759947d6fdfc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0900000000-2d1db7a658b60e2c26b0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ac3-2900000000-a56eee18f6344558086e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0900000000-ac8c09535ca462176277 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0900000000-ac8c09535ca462176277 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-0900000000-3df3ee80cac7cf72baf3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-1900000000-f8e8dbfa16fdccfee793 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-002f-9300000000-0382b703ea178081272e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-054o-9300000000-93fdd27b1d13a2c799fb | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036683 |
|---|
| FooDB ID | FDB015613 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00021911 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 460835 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 528761 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|