| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:48:12 UTC |
|---|
| Update Date | 2016-11-09 01:19:03 UTC |
|---|
| Accession Number | CHEM030301 |
|---|
| Identification |
|---|
| Common Name | Furanofukinin |
|---|
| Class | Small Molecule |
|---|
| Description | Furanofukinin is found in giant butterbur. Furanofukinin is a constituent of Petasites japonicus (sweet coltsfoot). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
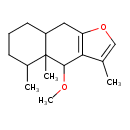
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 6b-Methoxyfuranoeremophilane | HMDB | | 6beta-Methoxyfuranoeremophilane | HMDB | | Petasalbin methyl ether | HMDB |
|
|---|
| Chemical Formula | C16H24O2 |
|---|
| Average Molecular Mass | 248.361 g/mol |
|---|
| Monoisotopic Mass | 248.178 g/mol |
|---|
| CAS Registry Number | 34335-93-8 |
|---|
| IUPAC Name | 4-methoxy-3,4a,5-trimethyl-4H,4aH,5H,6H,7H,8H,8aH,9H-naphtho[2,3-b]furan |
|---|
| Traditional Name | 4-methoxy-3,4a,5-trimethyl-4H,5H,6H,7H,8H,8aH,9H-naphtho[2,3-b]furan |
|---|
| SMILES | COC1C2=C(CC3CCCC(C)C13C)OC=C2C |
|---|
| InChI Identifier | InChI=1S/C16H24O2/c1-10-9-18-13-8-12-7-5-6-11(2)16(12,3)15(17-4)14(10)13/h9,11-12,15H,5-8H2,1-4H3 |
|---|
| InChI Key | JRLLKNNCOWNIBL-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as eremophilane, 8,9-secoeremophilane and furoeremophilane sesquiterpenoids. These are sesquiterpenoids with a structure based either on the eremophilane skeleton, its 8,9-seco derivative, or the furoeremophilane skeleton. Eremophilanes have been shown to be derived from eudesmanes by migration of the methyl group at C-10 to C-5. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesquiterpenoids |
|---|
| Direct Parent | Eremophilane, 8,9-secoeremophilane and furoeremophilane sesquiterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Furoeremophilane sesquiterpenoid
- Naphthofuran
- Benzofuran
- Heteroaromatic compound
- Furan
- Oxacycle
- Organoheterocyclic compound
- Ether
- Dialkyl ether
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05o0-2390000000-e1dfa66e24b593c7990f | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0090000000-b127a46b3ae53aa9de38 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00r2-3490000000-4e6d1b166e07e3d2aca7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gbc-9310000000-e5cd56e200170ab66737 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-0d95bd14aca56b2622aa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0090000000-9e2dffa207fbb394051f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0uyi-1980000000-06e09aa335cd04fc1d1f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-2ee4b8ddcdaa7b3755dc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0090000000-3bb81aed3f9a7fc6b757 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0lmj-3940000000-c040ca173f64a00c9032 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0090000000-d470e68b398edadf6917 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0490000000-3bb74306ed0852d1c737 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-6940000000-75d86a1892ed5c28fc65 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036640 |
|---|
| FooDB ID | FDB015560 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00017371 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35014176 |
|---|
| ChEBI ID | 168175 |
|---|
| PubChem Compound ID | 78385403 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|