| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:47:59 UTC |
|---|
| Update Date | 2016-11-09 01:19:03 UTC |
|---|
| Accession Number | CHEM030296 |
|---|
| Identification |
|---|
| Common Name | Hydroxytanshinone |
|---|
| Class | Small Molecule |
|---|
| Description | Minor constituent of Salvia sclarea (clary sage). Hydroxytanshinone is found in tea, alcoholic beverages, and herbs and spices. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
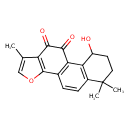
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Hydroxy-14,16-epoxy-20-nor-5(10),6,8,13,15-abietapentaene-11,12-dione | HMDB |
|
|---|
| Chemical Formula | C19H18O4 |
|---|
| Average Molecular Mass | 310.344 g/mol |
|---|
| Monoisotopic Mass | 310.121 g/mol |
|---|
| CAS Registry Number | 18887-18-8 |
|---|
| IUPAC Name | 3-hydroxy-6,6,14-trimethyl-12-oxatetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadeca-1(10),2(7),8,11(15),13-pentaene-16,17-dione |
|---|
| Traditional Name | 3-hydroxy-6,6,14-trimethyl-12-oxatetracyclo[8.7.0.0²,⁷.0¹¹,¹⁵]heptadeca-1(10),2(7),8,11(15),13-pentaene-16,17-dione |
|---|
| SMILES | CC1=COC2=C1C(=O)C(=O)C1=C2C=CC2=C1C(O)CCC2(C)C |
|---|
| InChI Identifier | InChI=1S/C19H18O4/c1-9-8-23-18-10-4-5-11-15(12(20)6-7-19(11,2)3)14(10)17(22)16(21)13(9)18/h4-5,8,12,20H,6-7H2,1-3H3 |
|---|
| InChI Key | UPCWCCRFMPIOAP-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tanshinones, isotanshinones, and derivatives. These are a group of abietane-type norditerpenoid quinones. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Diterpenoids |
|---|
| Direct Parent | Tanshinones, isotanshinones, and derivatives |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | Not Available |
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001i-0190000000-303b948cd84ab5b3c3c8 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00y0-4039000000-f23fce8c9e528aaac142 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01ox-0095000000-1459090115953e2d8ada | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-1091000000-9b160e8f70ba1ebd0e8c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0v4l-4290000000-673a83937492b73d2faf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0029000000-857494f71efc4fd3d067 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0069000000-e26deb1c0ad2f400236e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004j-0190000000-fc4e16c2c20e31bada31 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0029000000-fac4878c9215b2cd3161 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4l-0069000000-4fb07203823f1c3c868f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004l-0191000000-b9d77947a2efacfb4303 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0049000000-095cdc9f44d9d61d2906 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-040r-0091000000-91de6cd8c46488f5e66a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ue9-0090000000-cd483d43e8533af44a90 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036635 |
|---|
| FooDB ID | FDB015555 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4476947 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 5318349 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|