| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:47:00 UTC |
|---|
| Update Date | 2016-11-09 01:19:02 UTC |
|---|
| Accession Number | CHEM030273 |
|---|
| Identification |
|---|
| Common Name | Pterosin F |
|---|
| Class | Small Molecule |
|---|
| Description | Pterosin F is found in green vegetables. Pterosin F is a constituent of Pteridium aquilinum (bracken fern). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
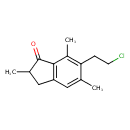
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 6-(2-Chloroethyl)-2,3-dihydro-2,5,7-trimethyl-1H-inden-1-one, 9ci | HMDB | | 6-(2-Chloroethyl)-2,5,7-trimethyl-(-)-1-indanone | HMDB |
|
|---|
| Chemical Formula | C14H17ClO |
|---|
| Average Molecular Mass | 236.737 g/mol |
|---|
| Monoisotopic Mass | 236.097 g/mol |
|---|
| CAS Registry Number | 34175-98-9 |
|---|
| IUPAC Name | 6-(2-chloroethyl)-2,5,7-trimethyl-2,3-dihydro-1H-inden-1-one |
|---|
| Traditional Name | 6-(2-chloroethyl)-2,5,7-trimethyl-2,3-dihydroinden-1-one |
|---|
| SMILES | CC1CC2=C(C1=O)C(C)=C(CCCl)C(C)=C2 |
|---|
| InChI Identifier | InChI=1S/C14H17ClO/c1-8-6-11-7-9(2)14(16)13(11)10(3)12(8)4-5-15/h6,9H,4-5,7H2,1-3H3 |
|---|
| InChI Key | DFJCTWMNTSWCRI-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as indanones. Indanones are compounds containing an indane ring bearing a ketone group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Indanes |
|---|
| Sub Class | Indanones |
|---|
| Direct Parent | Indanones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Indanone
- Aryl alkyl ketone
- Aryl ketone
- Ketone
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organochloride
- Organohalogen compound
- Alkyl halide
- Alkyl chloride
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0kg9-2950000000-f663ddf4cc75aa110084 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0290000000-c1f23dbd4cb2efd1cd36 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-1790000000-0729005f85a1582da512 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05fr-2900000000-6dc4dc31de048cd7bc11 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0190000000-254cf49aa630fe91d0de | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000j-0690000000-5a363c889f113b494327 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4j-3930000000-f1a8bb188f7bfcdee521 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-60ac5975c4de9e8110db | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0019-6490000000-e121a85d00b4149f4f89 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-0920000000-e6295b2c6717df809143 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0090000000-ce0fdf152dbb21b79827 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0590000000-c967a3656826a8b83cc8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0bu9-0900000000-98032b2689a32052df1e | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036606 |
|---|
| FooDB ID | FDB015522 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00055535 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 118944 |
|---|
| ChEBI ID | 169882 |
|---|
| PubChem Compound ID | 134978 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|