| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:44:58 UTC |
|---|
| Update Date | 2016-11-09 01:19:02 UTC |
|---|
| Accession Number | CHEM030227 |
|---|
| Identification |
|---|
| Common Name | Cyclolinopeptide G |
|---|
| Class | Small Molecule |
|---|
| Description | Cyclolinopeptide G is found in fats and oils. Cyclolinopeptide G is a constituent of the seeds of Linum usitatissimum (flax). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
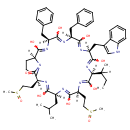
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (3S,6S,9S,12S,15S,18S,21S,26AS)-3,6-dibenzyl-12-(butan-2-yl)-1,4,7,10,13,16,19-heptahydroxy-9-[(1H-indol-3-yl)methyl]-15,21-bis(2-methanesulphinylethyl)-18-(2-methylpropyl)-3H,6H,9H,12H,15H,18H,21H,22H,24H,25H,26H,26ah-pyrrolo[1,2-a]1,4,7,10,13,16,19,22-octaazacyclotetracosan-22-one | HMDB |
|
|---|
| Chemical Formula | C56H75N9O10S2 |
|---|
| Average Molecular Mass | 1098.379 g/mol |
|---|
| Monoisotopic Mass | 1097.508 g/mol |
|---|
| CAS Registry Number | 351417-16-8 |
|---|
| IUPAC Name | (3S,6S,9S,12S,15S,18S,21S,26aS)-3,6-dibenzyl-12-(butan-2-yl)-9-(1H-indol-3-ylmethyl)-15,21-bis(2-methanesulfinylethyl)-18-(2-methylpropyl)-hexacosahydropyrrolo[1,2-a]1,4,7,10,13,16,19,22-octaazacyclotetracosan-1,4,7,10,13,16,19,22-octone |
|---|
| Traditional Name | (3S,6S,9S,12S,15S,18S,21S,26aS)-3,6-dibenzyl-9-(1H-indol-3-ylmethyl)-15,21-bis(2-methanesulfinylethyl)-18-(2-methylpropyl)-12-(sec-butyl)-octadecahydropyrrolo[1,2-a]1,4,7,10,13,16,19,22-octaazacyclotetracosan-1,4,7,10,13,16,19,22-octone |
|---|
| SMILES | CCC(C)[C@@H]1NC(=O)[C@H](CC2=CNC3=CC=CC=C23)NC(=O)[C@H](CC2=CC=CC=C2)NC(=O)[C@H](CC2=CC=CC=C2)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CCS(C)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCS(C)=O)NC1=O |
|---|
| InChI Identifier | InChI=1S/C56H75N9O10S2/c1-7-35(4)48-55(72)58-41(24-27-76(5)74)49(66)60-43(29-34(2)3)50(67)59-42(25-28-77(6)75)56(73)65-26-16-23-47(65)54(71)63-45(31-37-19-12-9-13-20-37)52(69)61-44(30-36-17-10-8-11-18-36)51(68)62-46(53(70)64-48)32-38-33-57-40-22-15-14-21-39(38)40/h8-15,17-22,33-35,41-48,57H,7,16,23-32H2,1-6H3,(H,58,72)(H,59,67)(H,60,66)(H,61,69)(H,62,68)(H,63,71)(H,64,70)/t35?,41-,42-,43-,44-,45-,46-,47-,48-,76?,77?/m0/s1 |
|---|
| InChI Key | MGTCVHJATWUGDT-VAFFMOIVSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Oligopeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-oligopeptide
- Cyclic alpha peptide
- Macrolactam
- Alpha-amino acid or derivatives
- 3-alkylindole
- Indole
- Indole or derivatives
- Monocyclic benzene moiety
- Benzenoid
- Substituted pyrrole
- Heteroaromatic compound
- Pyrrole
- Pyrrolidine
- Tertiary carboxylic acid amide
- Carboxamide group
- Lactam
- Secondary carboxylic acid amide
- Sulfoxide
- Azacycle
- Organoheterocyclic compound
- Sulfinyl compound
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic nitrogen compound
- Organic oxygen compound
- Carbonyl group
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-9000000000-81219a6c143dc7fa893f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-9000000000-e2c96a78a1ad76181168 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03di-9000000000-d9b6a329012dd852bae0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000t-9000000000-c062a28419ee37878bb7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-9000000000-2a0d8a68d4eea645db08 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00e9-9100000005-9b83592843d2561ac64b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-9000000000-b47591d465c4a05968b8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-016s-9100000017-a91daf394653af53f18e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-003u-6900000023-5a068a4402092ed51b73 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-9000000000-fbd25f74cd409469ccd0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000t-9000000000-e97f003e846347799b5b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-9600000000-7257a373be11b2885d22 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036551 |
|---|
| FooDB ID | FDB015454 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00027044 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4911169 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 6398539 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|