| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:43:46 UTC |
|---|
| Update Date | 2016-11-09 01:19:02 UTC |
|---|
| Accession Number | CHEM030203 |
|---|
| Identification |
|---|
| Common Name | Achillicin |
|---|
| Class | Small Molecule |
|---|
| Description | Achillicin is found in herbs and spices. Achillicin is a constituent of Achillea millefolium (yarrow). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
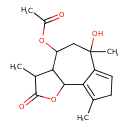
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2,2-dichloro-N-Methyl-acetamide | HMDB | | 2,2-dichloro-N-Methylacetamide | HMDB | | 8-Acetoxyartabsin | HMDB | | 6-Hydroxy-3,6,9-trimethyl-2-oxo-2H,3H,3ah,4H,5H,6H,8H,9BH-azuleno[4,5-b]furan-4-yl acetic acid | Generator |
|
|---|
| Chemical Formula | C17H22O5 |
|---|
| Average Molecular Mass | 306.354 g/mol |
|---|
| Monoisotopic Mass | 306.147 g/mol |
|---|
| CAS Registry Number | 71616-00-7 |
|---|
| IUPAC Name | 6-hydroxy-3,6,9-trimethyl-2-oxo-2H,3H,3aH,4H,5H,6H,8H,9bH-azuleno[4,5-b]furan-4-yl acetate |
|---|
| Traditional Name | 6-hydroxy-3,6,9-trimethyl-2-oxo-3H,3aH,4H,5H,8H,9bH-azuleno[4,5-b]furan-4-yl acetate |
|---|
| SMILES | CC1C2C(OC1=O)C1=C(C)CC=C1C(C)(O)CC2OC(C)=O |
|---|
| InChI Identifier | InChI=1S/C17H22O5/c1-8-5-6-11-13(8)15-14(9(2)16(19)22-15)12(21-10(3)18)7-17(11,4)20/h6,9,12,14-15,20H,5,7H2,1-4H3 |
|---|
| InChI Key | ZPTLTKQKVWFFNY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as gamma butyrolactones. Gamma butyrolactones are compounds containing a gamma butyrolactone moiety, which consists of an aliphatic five-member ring with four carbon atoms, one oxygen atom, and bears a ketone group on the carbon adjacent to the oxygen atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Lactones |
|---|
| Sub Class | Gamma butyrolactones |
|---|
| Direct Parent | Gamma butyrolactones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Gamma butyrolactone
- Dicarboxylic acid or derivatives
- Tetrahydrofuran
- Tertiary alcohol
- Cyclic alcohol
- Carboxylic acid ester
- Oxacycle
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00fu-5890000000-4c01d3deb7b1eed29bc3 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-002f-7869000000-9fd0ad439d09106adf07 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0bt9-1096000000-16a539f456aa419f52a3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0bta-2091000000-a41577dbcc3177a23e23 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4r-9660000000-0d5f1d51eaac0be0be8e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0090000000-b1574c55be79e83ef64b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01ot-1090000000-9bfaec8e1f32279da492 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a6u-9360000000-71225273d00873fc6811 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052r-0092000000-355fb2eace891efd3544 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014s-1290000000-bb73a688a3d500ca3fce | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udl-9350000000-603410c2d4b48a58873e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0191000000-20154b664ebc9cb01276 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-3392000000-eed430f0d7ce0656856b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0007-9240000000-ac794cc0b5be26ee8e68 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036487 |
|---|
| FooDB ID | FDB015382 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00020476 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35014157 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131752003 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|