| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:42:31 UTC |
|---|
| Update Date | 2016-11-09 01:19:01 UTC |
|---|
| Accession Number | CHEM030179 |
|---|
| Identification |
|---|
| Common Name | 1,3,7,9-Tetramethyluric acid |
|---|
| Class | Small Molecule |
|---|
| Description | An oxopurine that is uric acid in which the hydrogens at positions 1,3,7 and 9 are replaced by methyl groups. It is a purine alkaloid that is found in Chinese tea known as kucha (Camellia assamica var. kucha) and exhibits anti-inflammatory and analgesic properties. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
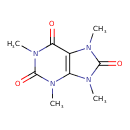
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Ba 2750 | ChEBI | | TeaCrine | ChEBI | | Temorine | ChEBI | | Tetramethyl uric acid | ChEBI | | Tetramethyl-2,3,6,7,8,9-hexahydro-1H-purine-2,6,8-trione | ChEBI | | Tetramethyluric acid | ChEBI | | Theacrine | ChEBI | | Tetramethyl urate | Generator | | Tetramethylate | Generator | | Tetramethylic acid | Generator | | 1,3,7,9-Tetramethyluric acid | MeSH | | 1379-Tetramethyluric acid | ChEMBL, HMDB | | 1379-Tetramethylate | Generator, HMDB | | 1379-Tetramethylic acid | Generator, HMDB | | 1,3,7, 9-Tetramethyluric acid | HMDB | | 1,3,7,9-Tetramethyl-7,9-dihydro-1H-purine-2,6,8(3H)-trione | HMDB | | 1,3,7,9-Tetramethylate | Generator | | 1,3,7,9-Tetramethylic acid | Generator |
|
|---|
| Chemical Formula | C9H12N4O3 |
|---|
| Average Molecular Mass | 224.217 g/mol |
|---|
| Monoisotopic Mass | 224.091 g/mol |
|---|
| CAS Registry Number | 2309-49-1 |
|---|
| IUPAC Name | tetramethyl-2,3,6,7,8,9-hexahydro-1H-purine-2,6,8-trione |
|---|
| Traditional Name | tetramethyluric acid |
|---|
| SMILES | CN1C(=O)N(C)C2=C1N(C)C(=O)N(C)C2=O |
|---|
| InChI Identifier | InChI=1S/C9H12N4O3/c1-10-5-6(11(2)8(10)15)12(3)9(16)13(4)7(5)14/h1-4H3 |
|---|
| InChI Key | QGDOQULISIQFHQ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as xanthines. These are purine derivatives with a ketone group conjugated at carbons 2 and 6 of the purine moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Imidazopyrimidines |
|---|
| Sub Class | Purines and purine derivatives |
|---|
| Direct Parent | Xanthines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Xanthine
- 6-oxopurine
- Purinone
- Alkaloid or derivatives
- Pyrimidone
- N-substituted imidazole
- Pyrimidine
- Azole
- Imidazole
- Heteroaromatic compound
- Vinylogous amide
- Lactam
- Urea
- Azacycle
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-014i-1910000000-620bbdc27e2d2ab3fa6f | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0090000000-8e9f489ece941b1ce61c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-016r-0950000000-342e6c57156137ef747d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-2900000000-76be8ee71d5baf16b79c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0190000000-8ba7d307c874a65578f1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0490000000-0fcaaaabdf4b0807a3ef | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0aor-3900000000-40d235f6bb5475c84b8e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0090000000-e02bcb3c0c3edf199294 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0390000000-a1803341d3d60eeb3370 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-2900000000-8259fb7d643d26f28bec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0090000000-579c7f6492ff0d502c20 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0910000000-ff3114d0e44ee51de61d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0670-1900000000-526309eb3fb97359b08e | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0004328 |
|---|
| FooDB ID | FDB015351 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00034312 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | CPD-12505 |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Theacrine |
|---|
| Chemspider ID | 67862 |
|---|
| ChEBI ID | 139388 |
|---|
| PubChem Compound ID | 75324 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|