| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:41:53 UTC |
|---|
| Update Date | 2016-11-09 01:19:01 UTC |
|---|
| Accession Number | CHEM030163 |
|---|
| Identification |
|---|
| Common Name | alpha-Bulnesene |
|---|
| Class | Small Molecule |
|---|
| Description | alpha-Bulnesene is found in cottonseed. alpha-Bulnesene is a constituent of guaiac wood oil (Bulnesia sarmienti). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
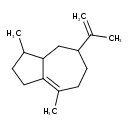
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| a-Bulnesene | Generator | | Α-bulnesene | Generator | | D-Guaiene | HMDB | | delta-Guaiene | HMDB | | Guaia-1(10),11-diene | HMDB | | laquo deltaraquo -Guaiene | HMDB | | laquo deltaraquo -Guaiene (= alpha-bulnesene) | HMDB | | laquo deltaraquo -Guaiene (alpha-bulnesene) | HMDB | | laquo deltaraquo -Guaijene | HMDB | | Guaia-9,11-diene | MeSH |
|
|---|
| Chemical Formula | C15H24 |
|---|
| Average Molecular Mass | 204.351 g/mol |
|---|
| Monoisotopic Mass | 204.188 g/mol |
|---|
| CAS Registry Number | 3691-11-0 |
|---|
| IUPAC Name | 3,8-dimethyl-5-(prop-1-en-2-yl)-1,2,3,3a,4,5,6,7-octahydroazulene |
|---|
| Traditional Name | 1,4-dimethyl-7-(prop-1-en-2-yl)-1,2,3,5,6,7,8,8a-octahydroazulene |
|---|
| SMILES | CC1CCC2=C(C)CCC(CC12)C(C)=C |
|---|
| InChI Identifier | InChI=1S/C15H24/c1-10(2)13-7-5-11(3)14-8-6-12(4)15(14)9-13/h12-13,15H,1,5-9H2,2-4H3 |
|---|
| InChI Key | YHAJBLWYOIUHHM-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sesquiterpenoids. These are terpenes with three consecutive isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesquiterpenoids |
|---|
| Direct Parent | Sesquiterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Sesquiterpenoid
- Branched unsaturated hydrocarbon
- Polycyclic hydrocarbon
- Cyclic olefin
- Unsaturated aliphatic hydrocarbon
- Unsaturated hydrocarbon
- Olefin
- Hydrocarbon
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01p9-2900000000-d2182918955206eed4fd | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0390000000-e6dbe7288e6337f129ed | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0bt9-3960000000-eabb188ee2a2084a0dcb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f7a-6900000000-3662a542c4b4f24d1556 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-2ae13e90f2534105ae4b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0190000000-5e0dce6bba1bc03af849 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0079-2900000000-8f1d37a0f1efc362abd1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-7ccf03fa1149a1e9f55f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0090000000-7ccf03fa1149a1e9f55f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-0390000000-fa796bcb7996f7793a6f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0290000000-06aacd79ae6b39209b53 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0592-7930000000-9a3587c868e5f2bb5d74 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0007-9200000000-3a135017e341fe806e85 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036444 |
|---|
| FooDB ID | FDB015331 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00020379 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 454293 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 520826 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|