| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 01:41:14 UTC |
|---|
| Update Date | 2016-11-09 01:19:01 UTC |
|---|
| Accession Number | CHEM030147 |
|---|
| Identification |
|---|
| Common Name | Persicachrome |
|---|
| Class | Small Molecule |
|---|
| Description | (3S,5R,8S)-Persicachrome is found in fruits. (3S,5R,8S)-Persicachrome is a constituent of flesh of cling peaches (Prunus persica). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
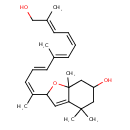
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (3S)-5,8-Epoxy-5,8-dihydro-12'-apo-beta,psi-carotene-3,12'-diol | HMDB | | 5,8-Epoxy-5,8-dihydro-12'-apo-b-carotene-3,12'-diol | HMDB |
|
|---|
| Chemical Formula | C25H36O3 |
|---|
| Average Molecular Mass | 384.552 g/mol |
|---|
| Monoisotopic Mass | 384.266 g/mol |
|---|
| CAS Registry Number | 80931-31-3 |
|---|
| IUPAC Name | 2-[(2Z,4E,6E,8Z,10E)-12-hydroxy-6,11-dimethyldodeca-2,4,6,8,10-pentaen-2-yl]-4,4,7a-trimethyl-2,4,5,6,7,7a-hexahydro-1-benzofuran-6-ol |
|---|
| Traditional Name | 2-[(2Z,4E,6E,8Z,10E)-12-hydroxy-6,11-dimethyldodeca-2,4,6,8,10-pentaen-2-yl]-4,4,7a-trimethyl-2,5,6,7-tetrahydro-1-benzofuran-6-ol |
|---|
| SMILES | C\C(CO)=C/C=C\C=C(/C)\C=C\C=C(\C)C1OC2(C)CC(O)CC(C)(C)C2=C1 |
|---|
| InChI Identifier | InChI=1S/C25H36O3/c1-18(10-7-8-11-19(2)17-26)12-9-13-20(3)22-14-23-24(4,5)15-21(27)16-25(23,6)28-22/h7-14,21-22,26-27H,15-17H2,1-6H3/b8-7-,12-9+,18-10+,19-11+,20-13- |
|---|
| InChI Key | MLVPRCYREVPVES-KWTPPHGWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as diterpenoids. These are terpene compounds formed by four isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Diterpenoids |
|---|
| Direct Parent | Diterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Diterpenoid
- Long chain fatty alcohol
- Benzofuran
- Fatty alcohol
- Fatty acyl
- Cyclic alcohol
- Dihydrofuran
- Secondary alcohol
- Dialkyl ether
- Oxacycle
- Organoheterocyclic compound
- Ether
- Alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0gb9-1419000000-dc948898e089f48e9a51 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-03di-4351390000-fb744d9d06a88e8c9717 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014r-0119000000-d68c3e85d61ea854b344 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-2947000000-b4ed3d4661c034c93c02 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0lk9-7910000000-5acf43e770021d464987 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0009000000-5edae22d1ccf26a56fd0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0159-0109000000-59c9549ccb9a979ee0d4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0gba-2639000000-4b04ac6830ab4e7b9477 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0170-0229000000-4764485fea6e8c6b1625 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00li-2944000000-c1cc5918b5cdd41ded6e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001l-2900000000-faf707b9ca9e33b6e6ef | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0009000000-df03b25fd2c880ff3c8a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0uyr-0009000000-eea02e151a40ae2d9f47 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000b-0098000000-4083a7c7669fc5cd774e | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0036425 |
|---|
| FooDB ID | FDB015312 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00023131 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35014147 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131751989 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|